Recently, we have reported on the potential of aerogel sorbents for CO2 capture and storage (CCS). Despite their favorable properties, the deployed amine functionalized aerogels (AMAs) were found to require optimization to allow for their successful economical implementation. Increasing the activity and capacity of solid sorbents while decreasing their cost, is therefore an issue which is currently under investigation. Researchers from the US and Sri Lanka now report to have found an efficient, cheap and environmentally benign solid CO2 sorbent: KOH-activated carbon chitin aerogels.
The novel sorbent material was synthesized from commercial chitin powder from shrimp shells, which was dispersed in a sodium-urea-water solution. Repeated freezing/thawing cycles of this solution resulted in the formation of a stable hydrogel, which subsequently was freeze-dried to obtain a chitin aerogel. Thereafter, carbonization of the aerogel was achieved by heating the sample to 800 °C under nitrogen atmosphere. In the last step, the aerogel was again heated to 850 °C (under N2 atmosphere) in the presence of potassium hydroxide (KOH) to obtain the activated carbon aerogel. Consequently, the inexpensive manufacturing technique, which does not require any costly or toxic chemicals, and the abundance of the precursor materials facilitate the cheap production of chitin-based CO2 sorbents.
The final activated carbon aerogels were found to exhibit large specific surface areas (> 500 m2/g), more than 35 times larger than that of their parent chitin aerogels. Additionally, the micro pore volume, which is an important parameter for CO2 capture, increased by the factor of 95 between the chitin aerogel and the carbonized and KOH-activated sample. These two factors explain why the obtained CO2 sorptivity value of 0.48 mmol/g (1 atm, 0 °C), obtained for the chitin aerogel, could be vastly increased to 5.02 mmol/g by further processing (i.e. carbonization and activation). As shown in the figure below, similar increase in sample sorptivity was also measured at room temperature (0.28 mmol/g and 3.44 mmol/g, respectively). This means that the morphological changes taking place inside the aerogel structure during carbonization and activation have a significant impact on the final sorbent properties.
The authors conclude that they have found an environmentally benign and very inexpensive way of manufacturing highly active chitin-based sorbents for CO2 capture. Additionally, the sorbents are synthesized from a biopolymer, making the final material biodegradable and non-toxic.
More details: Dassanayake, R.S., Gunathilake, C., Abidi, N. et al.; Activated carbon derived from chitin aerogels: preparation and CO2 adsorption, Cellulose (2018). https://doi.org/10.1007/s10570-018-1660-3

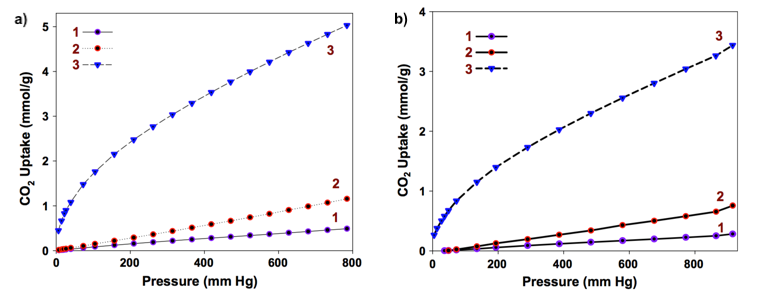 CO2 adsorption isotherms at 1 atm and 0 °C (a) and 1 atm and 25 °C (b) for chitin aerogels (1), carbonized chitin aerogels (2) and KOH-activated chitin aerogels
CO2 adsorption isotherms at 1 atm and 0 °C (a) and 1 atm and 25 °C (b) for chitin aerogels (1), carbonized chitin aerogels (2) and KOH-activated chitin aerogels