Strong and Flexible Aerogels
It’s true!
There are now many different types of aerogels that are flexible and high-strength, some of which are so mechanically robust they can actually be used for structural applications!
Aren’t Aerogels Already Superstrong?
Although it’s true that a typical silica aerogel could hold up to 2000 times its weight in applied force, this only holds if the force is gently and uniformly applied. Also, keep in mind that aerogels are also very light, and 2000 times the weight of an aerogel still might not be very much. Additionally, most aerogels as-produced are extremely brittle and friable (that is, they tend to fragment and pulverize). As a result, structural applications of aerogels were for a long time totally impractical.
But never fear! There are several ways aerogels can be made strong and even flexible, enough that aerogels can now be used as structural elements.
There are four general ways to enhance the mechanical properties of aerogels:
- Liquid-phase crosslinking
- Vapor-phase crosslinking
- Fiber reinforcing, and
- Reduced bonding
X-Aerogels: Crosslinked Aerogels
Particles of oxides, such as silica, are frequently mixed into plastics to make plastics with different properties. This process is called “doping”, in which the oxide particles are called a “filler”.
One day, Prof. Nicholas Leventis at the University of Missouri-Rolla (now the Missouri University of Science and Technology) wondered,
“If you can dope a polymer with a filler, can you dope a filler with a polymer?”
So thinking about cohesive forms of fillers used for doping polymers, he thought of silica aerogels, which are effectively macroscopic assemblies of silica nanoparticles.
Starting with a preformed wet silica gel of the type used for making silica aerogels, Leventis soaked the gel in solutions containing diisocyanates-crosslinking agents used to make polyurethane varnish-and then heated the gels to get the diisocyanates to bond. Upon supercritical drying, a silica aerogel with remarkably improved mechanical properties resulted-an aerogel that can actually bend not unlike stiff rubber! Try doing this with an ordinary silica aerogel and you’ll be left with lots of little broken pieces.
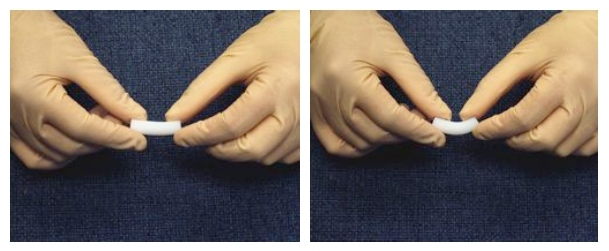
Silica x-aerogels exhibit rubber-like flexibility--not your great granddaddy's aerogel anymore! (Image courtesy Prof. Nicholas Leventis)
Diisocyanates are linear molecules with two ends that can react with hydroxyl groups to form carbamate bonds. The hydroxyl groups lining the skeleton of silica gels are perfect candidates for reacting with diisocyanates, and since diisocyanates have two reactive ends, they can bond to the aerogel skeleton twice. The result-each diisocyanate molecules acts like a nano-sized piece of Scotch® tape bonded to the surface of the aerogel skeleton, resulting in a conformal polymer skin that ties together the spherical silica nanoparticles that make up the aerogel. This conformal polymer skin makes the resulting aerogel much stronger than a typical silica aerogel and allows the structure to flex without breaking-sort of like the marshmallow coating on a Rice Krispies® treat!
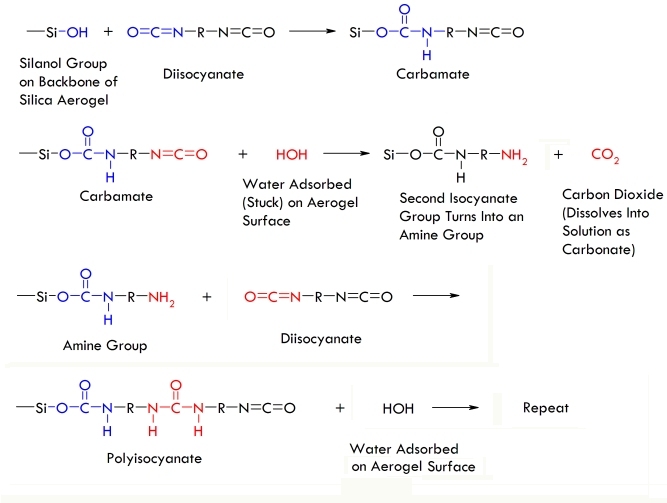
Chemical reactions that underly crosslinking of silica aerogels with a conformal polyisocyante coating
These strong, polymer-crosslinked aerogels are called “x-aerogels”. Importantly, the word x-aerogel (say “ex-air-o-jell”) is not the same thing as the word xerogel (say “zee-ro-jell”), which is a gel that has collapsed into a densified material as a result of evaporative drying.

X-aerogels are stiff and flexible, enabling them to withstand large deflections in a three-point bend test, a test that would ordinarily destroy a classic silica aerogel (image courtesy Prof. Nicholas Leventis)
How X-Aerogels Are Made
Pretty much any gel suitable for making aerogels can be modified to produce an x-aerogel. Here’s what has to happen:
- Make a gel. The gel can be made of silica, a transition metal oxide, an organic polymer or other substance.
- Identify a crosslinking agent that can react with surface sites on the gel. This typically means a polymer that will react with hydroxyl (-OH) groups to form a covalent bond, but for non-metal oxide gels it might be another functional group.
- Exchange the pore fluid in the gel with a solvent that will not react with your crosslinking agent through multiple soakings. For crosslinking agents that react with hydroxyl groups, this means something other than alcohol or water (which have hydroxyl groups on them themselves!) such as acetone or acetonitrile.
- Soak the gel in a solution of the new solvent with the crosslinking agent dissolved in it and, for some polymers, also containing a radical initiator. The amount of crosslinker put in will affect how heavily reinforced the aerogel will be.
- Allow enough time for the crosslinker solution to diffuse thoroughly into the gel. This will take at least 24 h for a small monolith and longer for pieces with lots of internal volume.
- In a sealed container, place the gel (still soaking under crosslinker solution) into an oven to thermally activate the crosslinking agent. The temperature of the oven depends on the crosslinking agent but typically a temperature of 60°C works well for polymers that react with hydroxyl groups on meal oxide gels.
- After 24-72 h, remove the gel from the oven and replace the crosslinker solution with fresh solvent.Finally,
- Solvent exchange into liquid CO2 and supercritically dry. Generally speaking, high-temperature supercritical drying won’t work with x-aerogels because the polymers will decompose at the conditions required for supercritical organic solvents.or alternatively,Solvent exchange into pentane or hexane and allow the gel to slowly evaporatively dry. Because of its conformal polymer coating, the crosslinked gel is much stronger than a typical gel used for making aerogels and can resist the capillary stress caused by evaporating that would cause an ordinary gel to collapse. The result-an x-aerogel without supercritical drying!
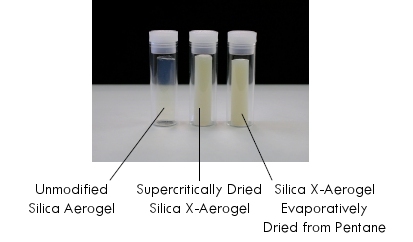
Comparison of x-aerogels prepared by supercritical drying and evaporative drying from pentane (image courtesy Prof. Nicholas Leventis)
Polymers Used for Making X-Aerogels
Lots of polymers can be used to crosslink aerogels, including:
- Polyisocyanates
- Epoxides
- Polystyrene
and lots more. To help these polymers attach to the gel framework, a compound called 3-aminopropyltriethoxysilane, or APTES for short, can be mixed in as the gel sets. This puts amine functional groups (-NH2) over the surface of the gel in addition to the hydroxyl groups that are normally there. These amine groups can be used to bond a wide variety of polymers to the gel framework. Dr. Mary Ann Meador at the NASA Glenn Research Center in Cleveland, Ohio has done extensive work refining this method.
Different Flavors of X-Aerogels
Since the crosslinking takes place separately from the gel formation step, you can crosslink pretty much any gel to make an x-aerogel. X-silica (crosslinked silica aerogel), x-alumina (crosslinked alumina aerogel), and x-RF (crosslinked resorcinol-formaldehyde polymer aerogel) are just a few that have been prepared. In fact, x-aerogels of almost all of the colorful lanthanide oxides have been made as well.
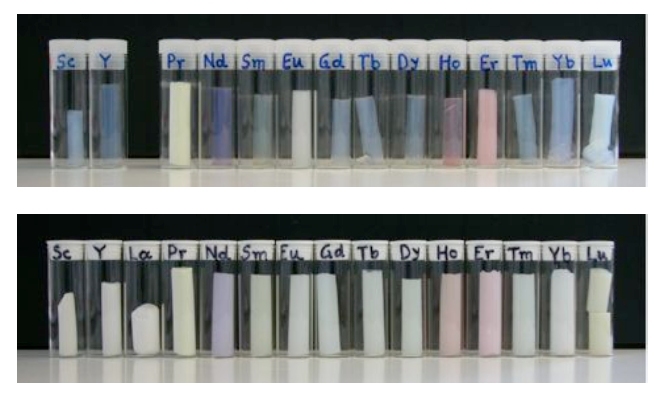
Virtually any metal oxide aerogel that can be made can be made in a mechanically strong x-aerogel form, including the colorful lanthanide oxides (image courtesy Prof. Nicholas Leventis)
Advantages and Disadvantages to Crosslinking Aerogels
So why isn’t every aerogel made today an x-aerogel? Well there are some trade-offs to consider.
Advantages:
- Dramatically increased strength
- Increased stiffness
- Flexibility
- Machineability and resistance to fracture
- Waterproof
- Impact resistance
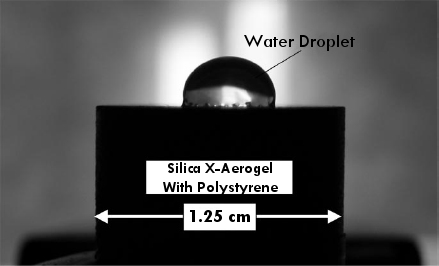
X-aerogels like this silica x-aerogel crosslinked with polystyrene are usually hydrophobic since the hydrophilic hydroxyl groups that would ordinarily line the aerogel backbone have beenreplaced by non-polar organic polymer chains (image courtesy Prof. Nicholas Leventis)
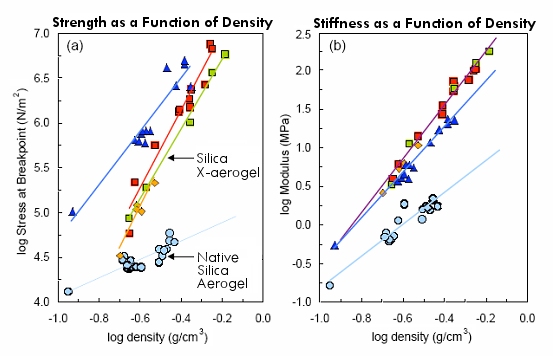
Compressive strength and stiffness (resistance to deflection) as a function of x-aerogel density (data courtesy Prof. Nicholas Leventis)
Disadvantages:
- Increased density (typically about one-third to one-half the density of water)
- Decreased clarity (from translucent to foggy to opaque)
- Decreased surface area (by about half)
- Reduction in insulating ability (from superinsulator to about as good as Styrofoam®)
- Requires more chemicals and usually more time
Applications for X-Aerogels
Because of their impressive strength-to-weight ratios, decent insulating ability, and nanostructural properties, there are some interesting applications for x-aerogels:
- Lightweight thermal insulation
- Lightweight acoustic insulation
- Insulating skylights
- Armor
- Run-flat tires
- Membranes for fuel cells
- Optical sensors
- Aircraft structural components
- Heat shields for spacecraft reentry
- Lightweight composite structures
X-aerogels are still new. Who knows what’s soon to come!
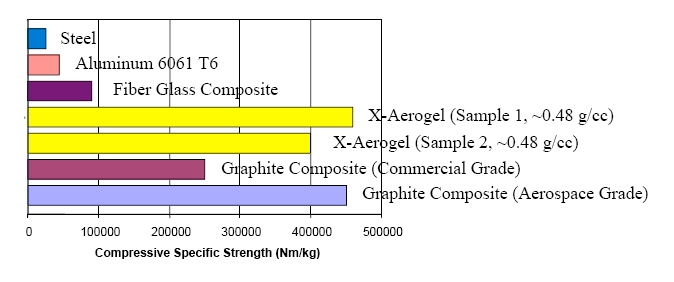
Compressive strength of x-aerogels compared to other materials (figure courtesy Prof. Nicholas Leventis)
Research on X-Aerogels
X-aerogel research is currently underway in the Leventis group at the Missouri University of Science and Technology in Rolla, MO and in the x-aerogel research group at NASA Glenn Research Center in Cleveland, OH led by Dr. Mary Ann Meador. Ongoing work to extend x-aerogel compositions to additional polymers, optimize processes for getting desired materials properties, and simplify and reduce cost of manufacture are currently underway.
Making X-Aerogels Through Vapor Deposition of “Nanosuperglue”
In addition to strengthening aerogels by crosslinking wet gels prior to supercritical drying, it’s possible to reinforce aerogels after they’ve been supercritically dried as well. This can be done through deposition of a conformal polymer coating throughout the interior porosity of an aerogel by means of chemical vapor deposition (CVD) or atomic layer deposition (ALD).
Boday et al. at Los Alamos National Laboratory demonstrated this principle through ambient-temperature CVD of methyl cyanoacrylate (the main ingredient in superglue) onto silica aerogels and found that the resulting conformal coating increased the strength of the aerogels 30-fold with only a three-fold increase in bulk density!
Based on the data for comparable untreated silica aerogels, this observed increase in strength due to the conformal coating is approximately three times greater than would be achieved by simply preparing a comparably dense aerogel using a more concentrated solution in preparing the precursor gel. Although they are made through a different way, these materials are also considered x-aerogels.
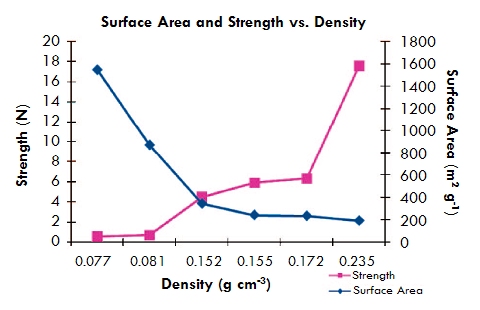
An increase in density of only three times due to deposition of methyl cyanoacrylate on a silica aerogel results in a 32 times increase in compressive strength--three times more than if you simply made the aerogel itself three times denser (adapted from Boday et al., Chemistry of Materials, 2008, 20 (9), 2847).
Fiber-Reinforced Aerogel Composite Blankets from Aspen Aerogels
Just like rebar reinforces concrete in bridges, it’s possible to reinforce aerogels with microfibers.
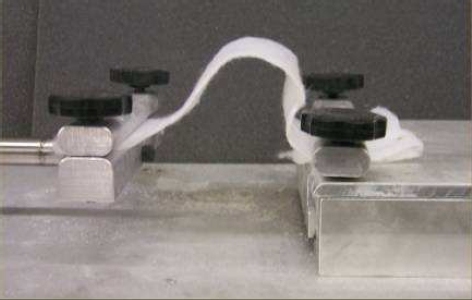
Aspen Systems, a contract research and development company, began experimenting with aerogels in the late 1990’s. Around 2000, they were experimenting with casting silica gel onto fibrous batting (a porous, flexible fiber mat) and supercritically drying to produce reinforced aerogels. To their surprise, the mat was totally flexible and as co-inventor Dr. George Gould described it, “almost as if it were a totally different material”. The flexible aerogel blanket showed to be almost as insulating as the plain aerogel, except for unlike a typical silica aerogel, it could be rolled up and bent over and over.
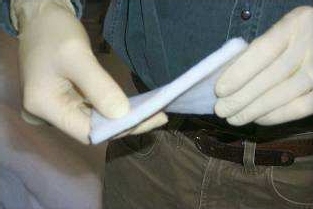
Flexible fiber-silica aerogel composite blankets made by Aspen Aerogels (images courtesy Aspen Aerogels)
In 2001, Aspen Aerogels spun off from Aspen Systems and since then has developed a range of aerogel blankets designed for different applications. Technically their materials are aerogel composites because they combine fibrous battings of inorganic or organic fibers with aerogels and, for high-temperature applications, carbon black as well. Numerous patents from Aspen detail production of mechanically robust, flexible aerogel blankets of both inorganic and organic aerogels supported by meshes of polyimides (i.e., Nylon®), glass fibers, and many other materials. Aspen’s products can be found in subsea oil pipelines, refineries, winter apparel, and even shoe insoles.
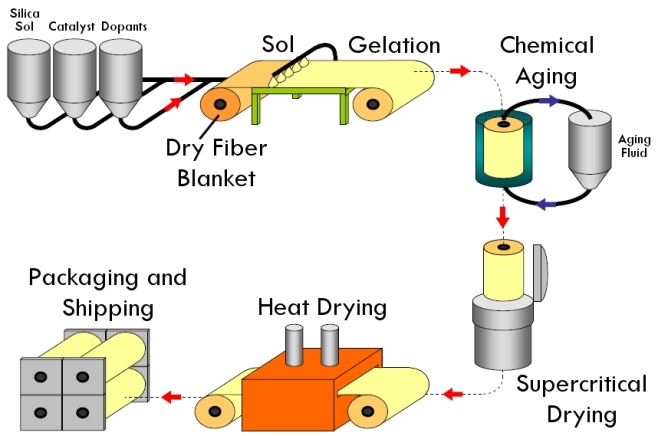
The blankets are made first by mixing up a sol as you would a normal silica aerogel. The sol is poured onto a roll of fibrous batting and heated until the gel sets. The mat is rolled up and then placed in a tank under liquid while the gel continues to set and strengthen and is made hydrophobic through chemical reactions. The roll is then moved into a giant supercritical dryer and supercritically dried from CO2. Finally, the roll is heated to drive off excess solvent and can be shipped out.
To the touch, aerogel blankets feel kind of like a soft Brillo Pad® and are a little crunchy in bending. The blankets give off a little bit of powder when handled, but as Aspen reports, the blankets can be flexed over 250,000 and lose only 2% mass. Handling the blankets will make your fingers feel slippery, as the dust from the aerogel is hydrophobic (water-repelling) and will stick to your fingers.
Despite the seemingly obvious potential of aerogels for all sorts of applications, it actually took quite a while for Aspen to figure out how to make a business of aerogel blankets. After exploring a number of markets and developing technologies for a number of applications with government funding, the killer app appeared-sub-sea oil pipelines. Slaggy oil carried in sub-sea pipelines needs to be kept warm to keep flowing, otherwise the cold temperatures of the surrounding water will freeze up the slag. To keep the oil warm, a “pipe-in-pipe” configuration is used, that is, an inner pipe surrounded by insulation in a larger outer pipe. Because of the thickness of polyurethane insulation required to keep the oil sufficiently warm, the outer pipe had to be large. And because of the size of the outer pipe required, only three ships in the world were capable of laying that kind of pipe. Enter flexible aerogel blankets. Because the thermal conductivity of aerogel blanket is about twice as low as polyurethane per unit thickness, a much thinner aerogel blanket can do the job of much thicker polyurethane, meaning the diameter of the outer pipe can be that much smaller. Suddenly, over 250 ships around the world could lay that diameter pipe, saving the oil industries billions of dollars!

Fiber-reinforced aerogel blankets have superior thermal insulating ability over polyurethane foam (PUF) (image courtesy Aspen Aerogels)
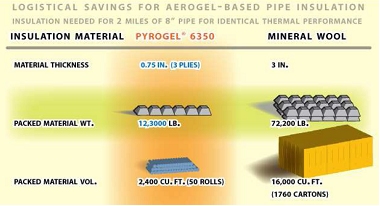
Fiber-reinforced aerogel blankets have superior thermal insulating ability over mineral wool, which makes them good for refineries (image courtesy Aspen Aerogels)
With that market in place, Aspen has started to address insulating needs in refineries and is working to replace mineral wool. Although today aerogel blankets are more expensive than other forms of insulation, they are not significantly more expensive and in the future with increased scale, aerogel will displace many types of insulation.
Aspen Aerogels aerogel blankets boast:
- Thermal conductivities as low as 0.014 W m-1 K-1
- Hydrophobicity
- Transpiration (they breathe)
- Flexibility
- Ability to be cut, sewn, and laminated
- Resistance to crushing and stepping on
- Simplified installation over other kinds of insulation
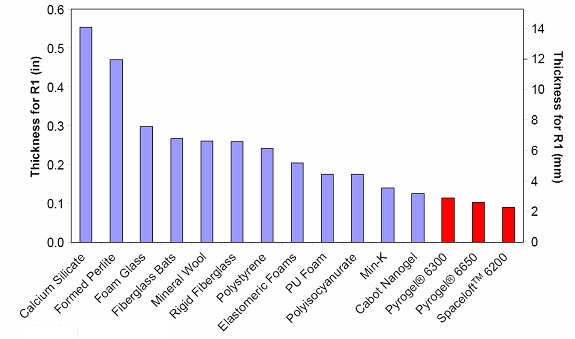
Different aerogel blanket formulations are designed for different applications. Cryogel® is designed for cryogenic applications, Spaceloft® is designed for clothing and apparel, and Pyrogel® is designed for high-temperature applications-six times better than mineral wool at temperatures of 350°C!
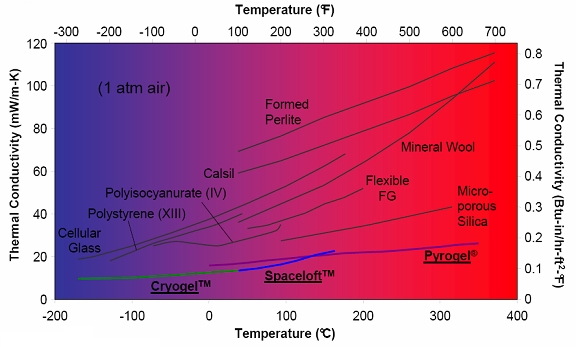
Thermal conductivity of various forms of insulation as a function of temperature (image courtesy Aspen Aerogels)
Aerogel blankets have many potential applications. One recent product is called Toasty Feet® and contains aerogel blanket in an insole for shoes to keep your feet warm in the winter and cool in the summer. Another product is aerogel blanket strips and aerogel wall wraps as home insulation that can help reduce heat loss through studs in the walls of a house.
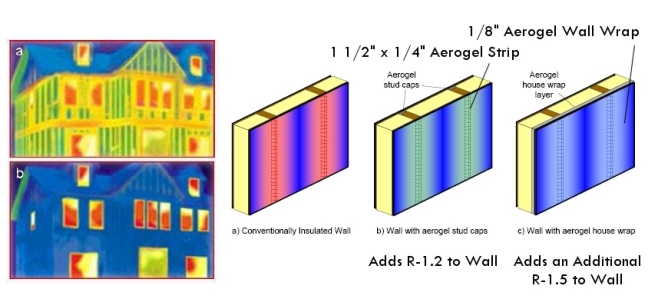
Strips and wraps of fiber-reinforced aerogel blankets could be used to improve insulation in the walls of a house (image courtesy Aspen Aerogels)
For more information about fiber-reinforced silica aerogel blankets, see US patents G. Gould, et al., United States Patent Application 20070222116, 2007, and J. Ryu, United States Patent 6,068,882, 2000.
Flexible, Superhydrophobic Silica Aerogels through Reduced Bonding
Prof. A. Venkateswara Rao (say “ven-cat-a-swar-a”) at Shivaji University in Kolhapur, India invented a really interesting way to make flexible silica aerogels that also strongly repel water-by reducing the amount of bonding in the aerogel!
Normally a silica aerogel is made with tetrafunctional silicon compounds such as TMOS (tetramethoxysilane), that is, compounds that will result in a silicon atom with four oxygen bridges connecting it to four other silicon atoms, with each of those silicon atoms having four oxygen bridges connecting them to four other silicon atoms, and so on and so forth. This makes a rigid structure in which each silicon atom is highly mechanically constrained.

Silica aerogels made with methyltrimethoxysilane exhibit reduced bonding compared to ordinary tetramethoxysilane-derived silica aerogels and are flexible (image courtesy Prof. A. Venketswara Rao)
But if you use a trifunctional silicon compound such as MTMS (methyltrimethoxysilane) instead, you get a structure in which each silicon atom only has three oxygen bridges to other silicon atoms and on the fourth bond (because silicon tends to form four bonds to stuff), one terminating methyl group that doesn’t connect to anything else. This makes a structure that has reduced overall bonding, with longer internal struts that are less mechanically constrained, which makes the overall aerogel flexible!
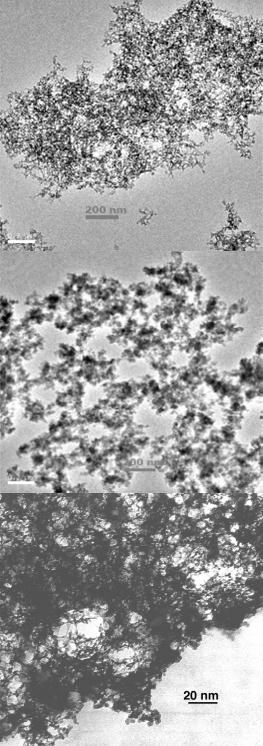
Aerogels made with reduced bonding (top and middle) exhibit a less constrained structure than aerogels made with tetrafunctional silane reagents like TMOS (bottom) (top and middle images courtesy Prof. A. Venkateswara Rao; bottom image credit Lawrence Livermore National Laboratory)
Water Floating on Water!
The methyl groups attached to each silicon atom in a flexible aerogel made with MTMS are highly hydrophobic, that is, repel water, and so where normal silica aerogels would have sticky, water-attracting hydroxyl groups on their surface, MTMS-based silica aerogels are highly water-repellant.
Mixing water with powdered silica aerogel made from MTMS and pouring the mixture out will result in an aerogel-coated liquid “marble”. Kind of like mixing oil and vinegar, the aerogel powder (which repels water like oil) separates out from the water, but because the droplet is small and has surface tension, the aerogel moves to the surface of the droplet and creates a shell around the water.
Here’s the wacky part-an aerogel-coated water droplet is itself water-repellant because of its aerogel shell, meaning it will float on water! That’s right, a water droplet that floats on water!
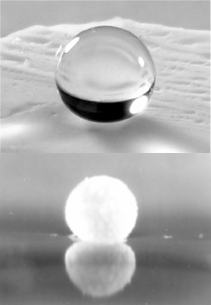
Silica aerogels made with reduced bonding are superhydrophobic and repel droplets of water due to highly non-polar methyl groups lining the skeletal surface (image courtesy Prof. A. Venkateswara Rao)
Combining both MTMS and TMOS, you can make silica aerogels with varying degrees of flexibility, optical transparency, and hydrophobicity.
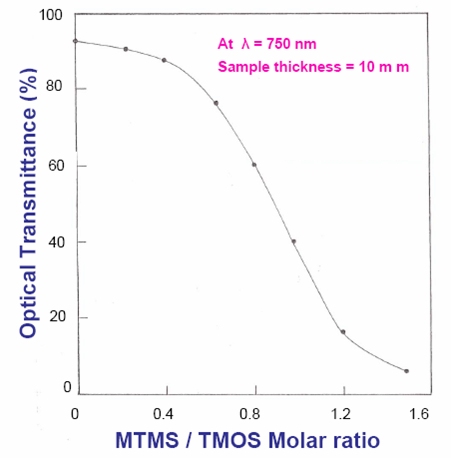
Optical transmission of flexible aerogels can be adjusted by combining silicon precursors (figure courtesy Prof. A. Venkateswara Rao)
This technique is called “reduced bonding” since compared to the typical way to make silica aerogel, each silicon atom makes fewer network bonds.
Applications of Reduced Bonding Aerogels
One exciting application for reduced bonding aerogels is absorption of toxic chemicals. The same thing that makes MTMS-derived silica aerogels water-repellant makes them good at absorbing non-polar organic compounds. MTMS-derived silica aerogels can absorb 13 times their weight in gasoline and 20 times their weight in toxic benzene!

Superhydrophobic aerogels absorb 10-20 times their weight in toxic, non-polar solvents such as benzene and gasoline (image courtesy Prof. A. Venkateswara Rao)
For more information about reduced bonding, see:
- A. V. Rao, G. M. Pajonk; J. Non-Crystalline Solids, 2001, 285, 202
- A. V. Rao, M. M. Kulkarni; J. Non-Crystalline Solids, 2003, 330, 187
- Rao, Pajonk, Bhagat, Philippe; J.Non- Crystalline Solids, 2004, 350, 216.



Nello, extremely interesting.
Anywhere one could buy x aerogel?
Thank you.
C
We’re testing Thermablok aerogel right now, which is a NASA-developed thermal break tape being used to short-circuit thermal leaks through metal stud framing in commercial construction. The Thermablok is applied to the metal studs prior to application of the exterior cladding system, is incompressible, and provides an R-value of 10.3 per inch of thickness. Their standard thickness is 3/8 inch, and it is a nice alternative to full foam board attached outside the studs, commonly known as EIFS, since foam production is a nasty process and it’s the thermal short-circuiting at the studs that’s the real problem
Fantastic article! In your opinion, how close are we to this material evolving into a Star Trek-like “Transparent Aluminum” or “Transparisteel” to be used in large structures or aviation? I was at JPL in Pasadena recently and saw that they already use aerogel-type materials in space exploration. It’s always exciting to see science-fiction turned into reality!
Great information! I’m thinking about using some forms of this material for a Product Design Project I’m working on. I’m thinking Household application indeed!
I would like to see a cost comparison chart in regards to other commonly used insulators. It’s probably here on the site somewhere. Maybe I hadn’t come across just yet.