Extraction and impregnation techniques employing supercritical CO2 as the working fluid can be considered as the non plus ultra in their fields, due to the extraordinary properties s.c. CO2 offers (e.g. high solubility, good miscibility, bio-compatibility, non-flammability, etc.). Especially in the field of aerogel synthesis supercritical drying techniques present the most favorable characteristics, yielding superior materials possessing large surface areas and porosities.
Yet, one major drawback of these processes are the high pressures needed during operation, necessitating expensive equipment (e.g. high pressure pumps) as well as extensive amounts of compression work, consequently entailing relatively high energy and plant costs.
To overcome this limitation of supercritical impregnation, extraction and drying techniques a team of scientists from the University of Birmingham (UK) have now suggested a novel cost-efficient plant setup
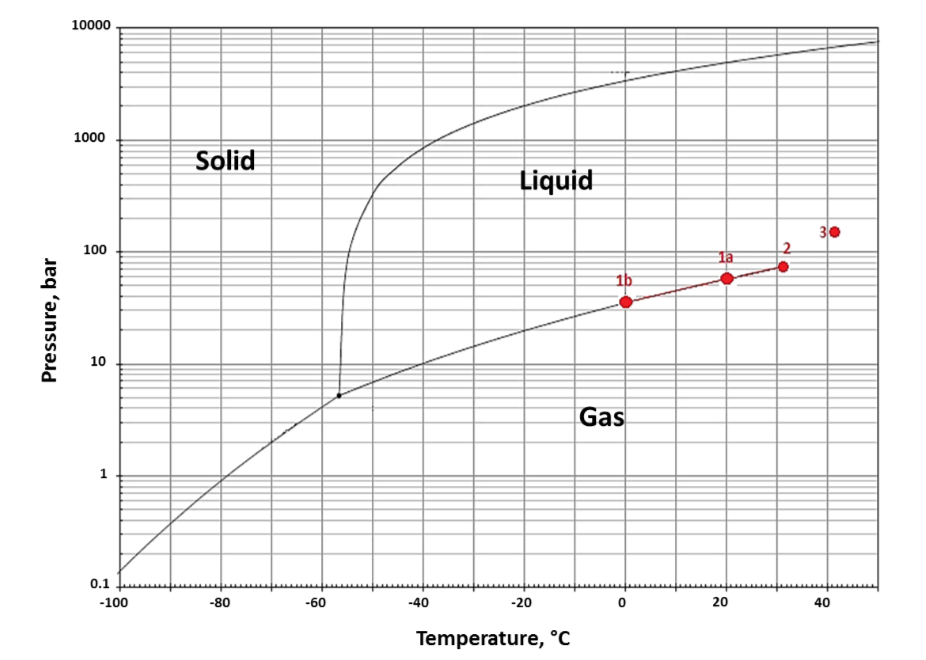
To achieve a plant setup not requiring any pumping or compression equipment, the authors suggest a relatively straight-forward approach — cooling down the pressure vessel (to 253 K/ -20 °C) prior to its filling with CO2. This simple preparation step led to the fact, that CO2 stored at ambient temperature, entered the vessel voluntarily when the inlet valve was being opened, due to the natural pressure gradient arising from the temperature difference (see figure below: 1a → 1b). As soon as the desired amount of CO2 was located inside the autoclave, it was sealed and subsequently heated to the desired final temperature, entailing an increase in system pressure and hence the obtainment of a supercritical fluid inside the vessel (see figure below: 1b → 3).
Consequently, a process not requiring any external pressurization equipment to pump CO2 into the setup or obtain the desired operating pressure was devised successfully.
To estimate the required final temperature and amount of CO2 inside the autoclave, which both have to be selected correctly for the process to work safely and properly, thermodynamic calculations based on a suitable equation of state (EOS) were conducted. Due to its suitability for supercritical fluids, the authors selected the Soave-Redlich-Kwong EOS for this purpose.
For the mapping and setting of both crucial parameters during operation, the setup was equipped with a temperature regulating unit and a high-resolution hanging scale attached to the autoclave.
Using the proposed setup, the authors were able to successfully dry monolithic LA gellan gum gels, impregnate freeze-dried gel structures with Vitamin E and extract caffeine from green coffee beans and black tea leaves. Furthermore, their economical analysis unveiled that the associated plant costs were reduced by a factor of 3 and 5, when compared to conventional batch and semicontinuous drying modes respectively, while the predicted energy costs were more than 30 % lower than for batch drying and 72 % lower than for semicontinuous drying.
Therefore, the authors concluded that their new plant setup is an auspicious alternative to conventional drying, impregnation and extraction configurations due to its flexibility and the lower investment and operation costs associated to it.
While these arguments may be true for small scale lab or tabletop configurations, for which the costs of the process periphery are relatively high and a broad applicability might be desired, it is dubious whether the same is true for larger, specialized configurations, for which the majority of costs generally stem from the autoclave. Especially the batch type mode of operation of the suggested technique can be considered as an exclusion criteria for industrial-scale plants, since it leads to dramatically longer processing times, vastly reducing the plant throughput.
A last concern of the novel process is its robustness and safeness, as small errors during operation (e.g. overfilling the autoclave or exceeding the desired temperature) can have devastating effects, turning the setup into a hazardous system. Since it will require a great deal of work to devise a fail-save plant, it is doubtful whether drying units deploying the suggested technique will ever be sold commercially.
More details: Cassanelli et al. “Design of a Cost-Reduced Flexible Plant for Supercritical Fluid-Assisted Applications” Chem. Eng. Technol. 2018, 41, No. 00, 1–11. https://doi.org/10.1002/ceat.201700487