The synthesis of soft and machinable, yet orderly porous materials has long been considered unfeasible. This is the case since in nature ordered porosities can be found in long-range extended crystalline networks, which are generally rigid and brittle, while softness and processability commonly originate from network defects and disorder in amorphous materials.
Researchers from Japan and Spain now report to have found a way to unite these properties once considered irreconcilable, paving the way for ultralight and flexible materials, which could find application in energy storage devices, building insulation and aerospace technology.
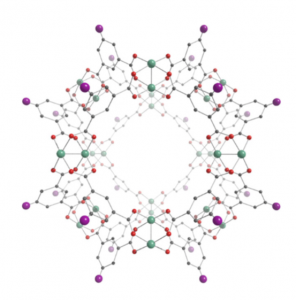
The very basis for the synthesis of this intriguing material are its small scale building blocks — so called metal-organic polyhedra (MOP) consisting of metal ions and ligands (see figure on the right). The
soluble, stable, permanently porous MOPs used by the researches consist of rhodium ions, oxygen and the so-called H2bdc-C12 ligand.
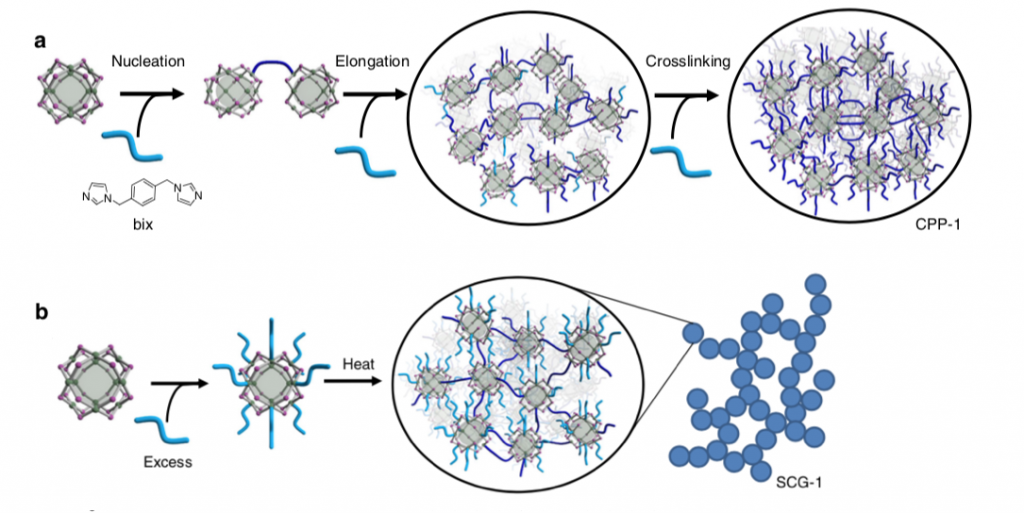
To achieve the coordination of the MOPs the researchers deployed a cross- linker molecule called 1,4-bis(imidazol-1-ylmethyl)benzene (for short: bix). The addition of this cross-linker resulted in the formation of coordination polymer particles (CPP) via a mechanism consisting of nucleation, elongation and cross-linking (see figure below). Remarkably, it was shown that an exact control of the resulting CPP size was possible through the adjustment of the deployed reaction conditions (e.g. amount of cross-linker added, rate of cross-linker addition). Moreover, the researchers found that the addition of excess bix followed by heating induces the formation of supramolecular colloidal gels (SCG). This means that, the intrinsic porosity of the MOPs was successfully integrated into two different large-scale amorphous geometries.
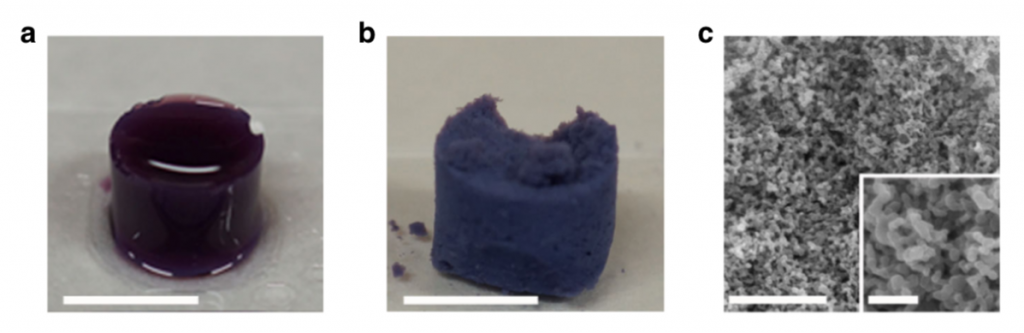
Through supercritical drying of the resulting SCGs with supercritical CO2, supramolecular aerogels (SAG) were obtained. These aerogels were found to exhibit a hierarchical macro-porous structure built up from fused polymer particles, while the intrinsic porosity of the MOP monomers was retained throughout the entire processing sequence. Consequently, aerogels possessing both micro and macro pores were synthesized (see figure below), leading to extraordinary adsorption properties of the final material (superior to blank MOPs and CPPs).
By fabricating two different macroscopic amorphous materials from monomeric MOPs (CPP and SCG/SAG), the authors found a way to better understand the process of synthesizing macroscopic shapes from molecular building blocks. On the basis of their remarkable findings, they assume that through further research on this topic, this “relationship between molecular scale geometries and resulting macroscopic shapes“ can be further investigated and eventually understood entirely, leading to groundbreaking advances “towards the development of soft matter that is both permanently porous and amenable to materials processing”.
More details: Carne-Sanchez et al. “Self-assembly of metal–organic polyhedra into supramolecular polymers with intrinsic microporosity ” Nature Communications Volume 9, https://www.nature.com/articles/s41467-018-04834-0
Read more at: https://phys.org/news/2018-07-stable-aerogels.html