Increasing atmospheric CO2 levels and dwindling fossil fuel resources have motivated the search for sustainable and renewable sources of energy. Hydrogen is considered to be an environmentally friendly alternative to common fossil fuels, as it does not emit environmentally harmful greenhouse gases upon combustion. Therefore, the quest for an efficient and sustainable way of generating hydrogen on a large scale is in full swing.
Supercritical water gasification (SCWG) of biomass is one such renewable hydrogen production technique that is currently being investigated. Due to the unique properties of supercritical water (e.g. high diffusivity, miscibility with gases) the process promises high energy conversion efficiencies. However, a suitable catalyst for the reaction, ensuring high H2 yields while suppressing coke and tar formation, has remained elusive. Researchers from the University of Western Ontario (Canada) have recently synthesized a Ru-Ni-Al2O3 catalyst which has shown promise for the SCWG process.
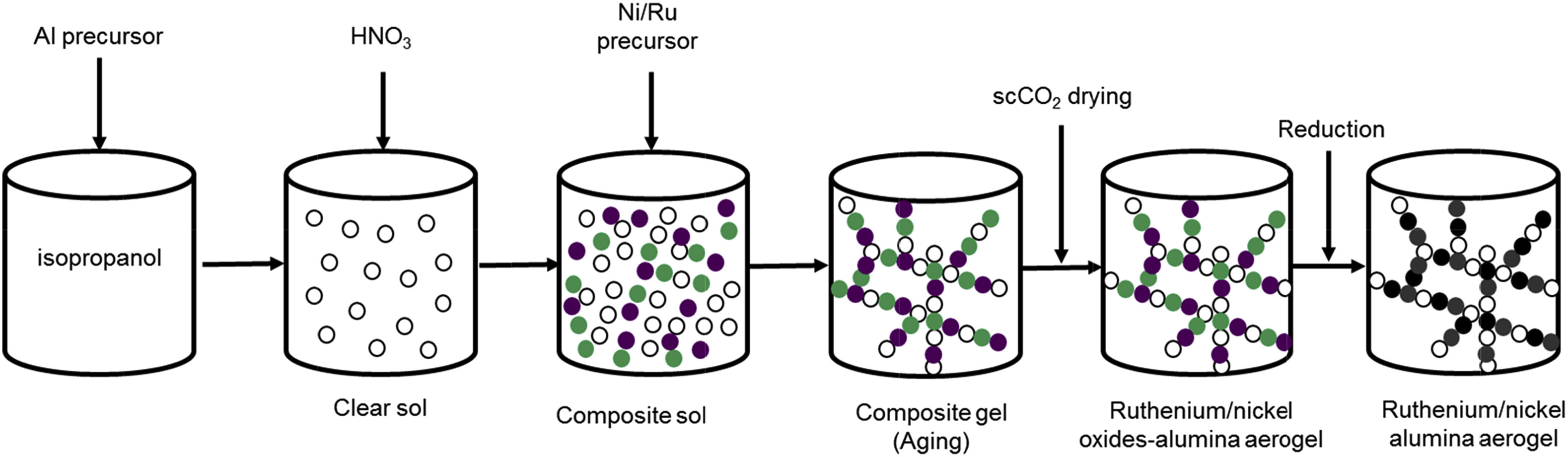
Synthesis of the catalyst required the formation of a clear solution which was achieved by mixing the aluminum support in isopropanol at 75 °C followed by the addition of nitric acid. Thereafter, the metallic precursors (nickel nitrate and ruthenium acetylacetonate) were added to the sol to initiate the aging process. After completion of the gel formation, the liquids contained in the porous structure were extracted via low temperature supercritical drying with CO2. In a last step the samples were calcined and reduced at 600 °C to obtain the ready-to-use aerogel catalysts (see Figure below).
The Ru-Ni-Al2O3 aerogel, synthesized via this process, exhibited a higher specific surface area and pore volume when compared to impregnated or xerogel Ru-Ni-Al2O3 catalysts. These superior structural features significantly enhanced the hydrogen yields during SCWG, due to the increase in available active surface area. Furthermore, the porous aerogel morphology was also shown to decrease unwanted coke formation on the catalyst surface. A comparison of Ni-Al2O3 and Ru-Ni-Al2O3 aerogel catalysts revealed that the promoting nature of ruthenium (Ru) leads to superior catalytic activities for the bimetallic composite. Additionally, the utilization of Ru further decreased coke formation during the gasification reaction.
In order to assess the cyclic stability of the aerogel structures Ni-Al2O3 and Ru-Ni-Al2O3 catalysts were employed in three consecutive SCWG reactions. Both structures showed signs of deactivation (e.g. decrease in surface area), however, even during the third experimental run a decent catalytic activity was observed for both structures. For example, the recycled Ru-Ni-Al2O3 aerogel exhibited only slightly smaller hydrogen yields than the fresh Ru-Ni-Al2O3 xerogel and the fresh impregnated Ni-Al2O3 catalysts.
Although numerous projects aiming at the development of renewable energy generation processes are in progress, we are still a long way from a fully formed strategy to replace fossil fuels globally. Therefore, technical advancements paving the way to a more sustainable future are essential. This study has shown that aerogels could play an integral role in propelling alternative processes such as supercritical water gasification to market maturity.
More details: Md. Zakir Hossain, Muhammad B.I. Chowdhury, Anil Kumar Jhawar, Paul A. Charpentier; Supercritical water gasification of glucose using bimetallic aerogel Ru-Ni-Al2O3 catalyst for H2 production, Biomass and Bioenergy Volume 107, December 2017, Pages 39-51. https://doi.org/10.1016/j.biombioe.2017.09.010