The search for alternative energy sources and novel means of (decentralized) power generation have become one of the central modern research topics. Due to their high efficiency and robustness fuel cells are considered to be an integral part in the envisaged future energy supply. Yet, conventional designs still require platinum-based catalysts to promote the oxygen reduction reaction (ORR). This has become a major obstacle in fuel cell technology, prohibiting their cost-efficient and widespread application. In light of this problem, the search for alternative catalyst materials has become a key aspect in fuel cell research.
Recent findings revealed that activated carbon (AC) is one such auspicious material for the catalysis of the ORR, which could replace conventional platinum (Pt) catalysts. Yet, AC exhibits significant shortfalls regarding its electrical conductivity as well as the number of active catalytic sites. Moreover, its application in fuel cells requires extremely large mass loadings to achieve decent performance.
To overcome these limitations, researchers from the Northwestern Polytechnical University, Xi’an (China) have suggested the production of AC-graphene hybrid aerogels as ORR catalyst materials.
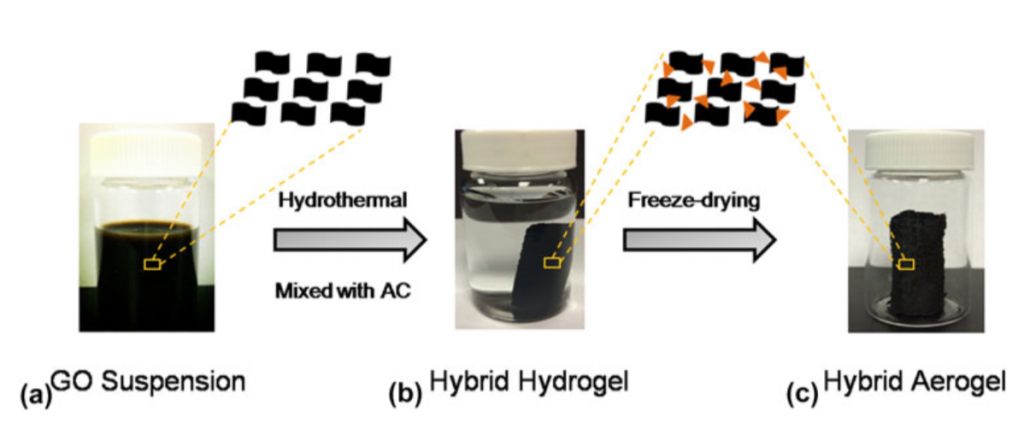
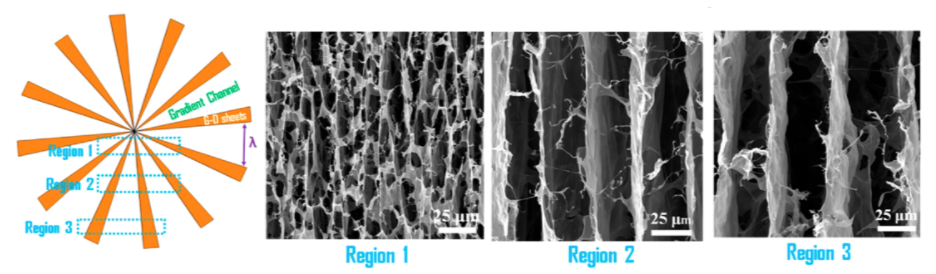
The composite aerogel materials were produced via the process schematically shown in the figure below, which includes the addition of AC to a graphene oxide dispersion, followed by hydrogel formation via hydrothermal processing and freeze drying.
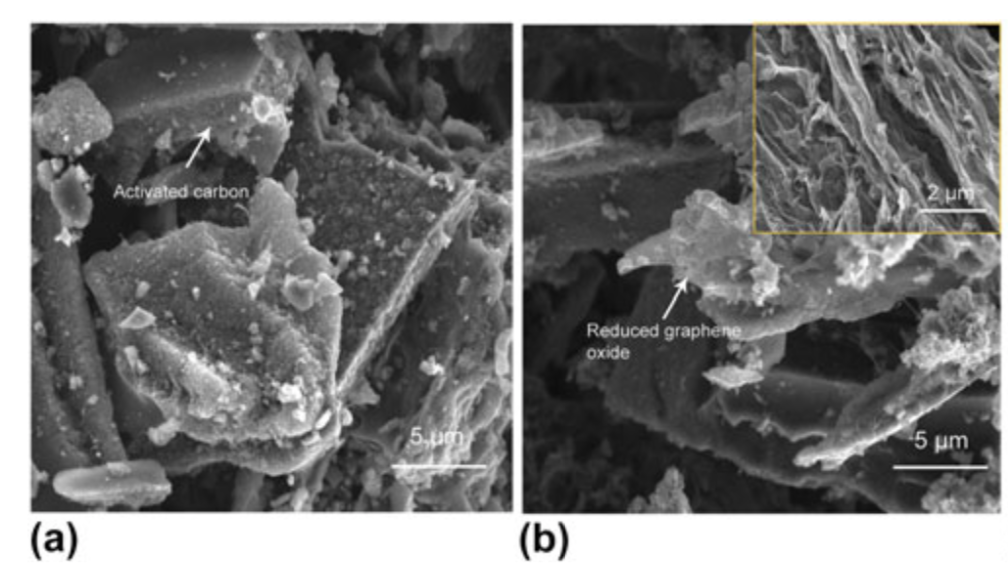
Characterization of the synthesized samples showed that through the addition of graphene to the aerogel matrix, the aerogel hybrids exhibited lower densities than pure AC (0.050-0.096 g/cm3), larger specific surface areas (500-750 m2/g) and a meso-porous structure consisting of more micro and meso pores than common activated carbon (see figure below).
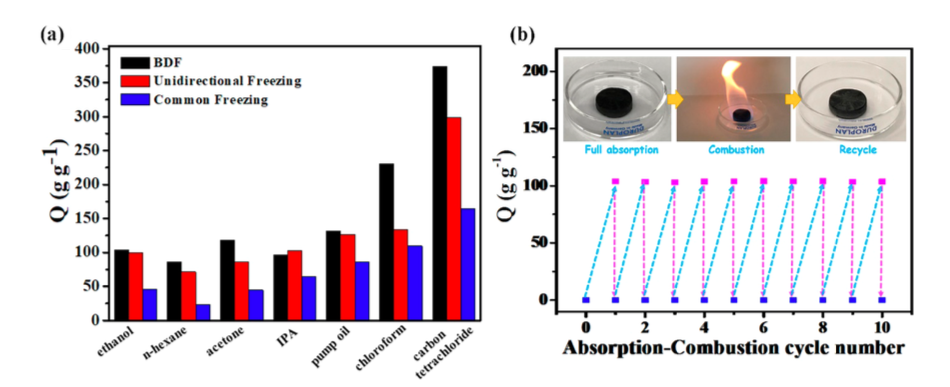
Owing to these superior morphological characteristics, the aerogel samples outperformed conventional AC in terms of the ORR catalytic performance (e.g. larger onset potential, limiting current density and exchange current density), which was shown in the course of multiple electrochemical measurements. These superior properties were validated by initial experiments in a electrolytic testing device, in which the AC-graphene aerogel electrode outperformed its plain AC counterpart at 20 times smaller mass loadings.
The authors conclude that the enhanced ORR performance at lower mass loadings can be attributed to the larger surface area and more micro-porous structure of AC-graphene aerogels when compared to pure AC. Since the suggested synthesis route can be scaled-up easily and does not include any expensive techniques or precursors, they see great potential in their new composite material, as it offers a cheap and scalable alternative for applications requiring light weight ORR catalysts (e.g. fuel cells or metal air batteries). Moreover, further enhancements in the hybrid’s electrochemical properties might be attained through doping the aerogel matrix with other atoms (e.g. N, S, P).
With fuel cells being one auspicious alternative to conventional power sources, technical progress in this field is required to reach a more sustainable future. Amongst others, this study shows that due to the extraordinary properties aerogel based materials offer they can help us to reach present or future political and societal milestones.
More details: Yang Yang and Honglong Chang “Multi-scale porous graphene/activated carbon aerogel enables lightweight carbonaceous catalysts for oxygen reduction reaction” Mater. Res. Vol. 33 No. 9 May 14 2018, https://doi.org/10.1557/jmr.2017.372



 The Association of Advanced Porous Materials (Advapor) is to offer a € 3000 award to the “best” PhD completed in the field of Advanced Porous Materials. Defense must occur before June 2019.
The Association of Advanced Porous Materials (Advapor) is to offer a € 3000 award to the “best” PhD completed in the field of Advanced Porous Materials. Defense must occur before June 2019.