Silica Aerogel
Silica aerogel is the most common type of aerogel. It is the type of aerogel most frequently seen in photographs and what people generally refer to when using the word “aerogel” without an adjective in front (although there are many other types of aerogel).
A Note About Silica vs. Silicon vs. Silicone
Silica refers to the oxide of silicon, which has the empirical formula SiO2. IT IS NOT SILICON. Silicon is a semiconductor metalloid used in microchips, whereas silica is an insulating glassy material. They are very different! Unfortunately, you will see “silicon aerogel” mentioned in the media from time to time (even on one of NASA’s websites!), however this is very incorrect, and should instead read “silica aerogel” (even Aerogel.org’s own co-founder Will Walker confuses the terms from time to time, but we forgive him). There are no reports of silicon aerogels yet, but they would certainly be interesting materials!
Silicones, on yet another hand, are polymers composed of silicon and oxygen, usually containing carbon and hydrogen as well. They are rubbery solids or liquids at room temperature and are very different from both silica and silicon. Think implants and temperature-resistant rubber. Silicone aerogels can be and have been prepared and possess many properties characteristic of silicone rubbers. This said, if someone says “silicone aerogel” they probably just mean “silica aerogel”.
Here’s a dumb pneumonic to help keep it straight: when you hear silicA think glAss, when you hear silicOn think cOmputers, and when you hear siliconE think rubbEr.
How is Silica Aerogel Made?
Like most other aerogels, silica aerogel starts its life out as a gel (see How Aerogel is Made). A silica gel serves as the precursor in this case and can be prepared through a number of chemical processes. It is important to note that the “silica gel” which comes in little packets marked “SILICA GEL DO NOT EAT” (usually found in consumer electronics packaging) is actually pellets of silica xerogel—a dense, dry, solid form of silica used to absorb moisture. Silica gels used for preparing silica aerogels, on the other hand, are wet gels close to gelatin dessert in consistency (but a bit crumblier). Silica gels are composed of two components—a solid, nanoporous silica-based framework which gives the gel its rigidity and solid form, and a liquid which permeates the pores of the framework.
Silica aerogel is made by extracting the liquid from the framework of the silica gel in a way that preserves at least 50% (but typically 90-99+%) of the gel framework’s original volume. This is typically done by supercritically drying the gel, but can also be done a number of other ways (see How Aerogel is Made).
Production of Silica Gels
Silica gels are produced through the sol-gel process, in which nanoparticles suspended in a liquid solution (i.e., a sol) are invoked to interconnect and form a continuous, porous, nanostructured network of particles across the volume of the liquid medium (i.e., a gel). See How Aerogel is Made for more information.
The Most Common Technique: Silicon Alkoxide Gelation
The most common technique used for producing silica gels today involves the reaction of a silicon alkoxide with water in a solvent such as ethanol or acetone, usually in the presence of basic, acidic, and/or fluoride-containing catalyst. In this technique, a silicon alkoxide (usually either tetramethoxysilane or tetraethoxysilane) serves as the source for the silica, water acts as a reactant to help join the alkoxide molecules together, and a catalyst (such as ammonium hydroxide or ammonium fluoride) helps the underlying chemical reactions go fast enough to be practically useful. Because silicon alkoxides are usually non-polar liquids, however, they are not miscible with water. As a result, a solvent such as ethanol or acetone, which is miscible with both silicon alkoxides and water, is added in order to get everything into the same phase so the necessary chemical reactions can occur.
In general, this type of chemistry can be done at ambient temperature and pressure simply by mixing the correct proportions of chemicals together—liquid goes in, gel comes out! Of course, different processes exist which are more complex this, each having their own advantages. Below is a walk-through of what happens at the molecular level when a silicon alkoxide reacts with water to form a silica gel.
A Note About Silicon Alkoxides
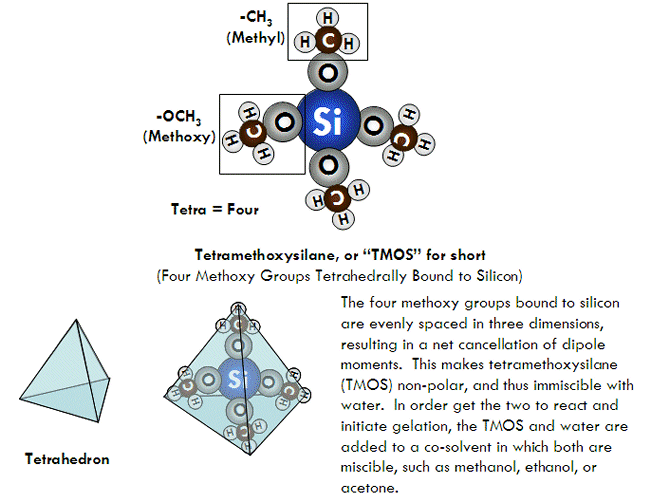
As mentioned, the most common type of silicon alkoxides used for making silica gels are tetramethoxysilane, abbreviated “TMOS”, and tetraethoxysilane, abbreviated “TEOS”. Both are tetrahedral, non-polar molecules. TMOS is also correctly called tetramethyl orthosilicate, where “orthosilicate” means SiO44-. Similarly, TEOS can also correctly be called tetraethyl orthosilicate. This is important to know, since some chemical companies use one name over the other. Other alkoxides, such as methyltrimethoxysilane (MTMS) are sometimes used for various reasons as well, which will be discussed later.
Formation of a Sol
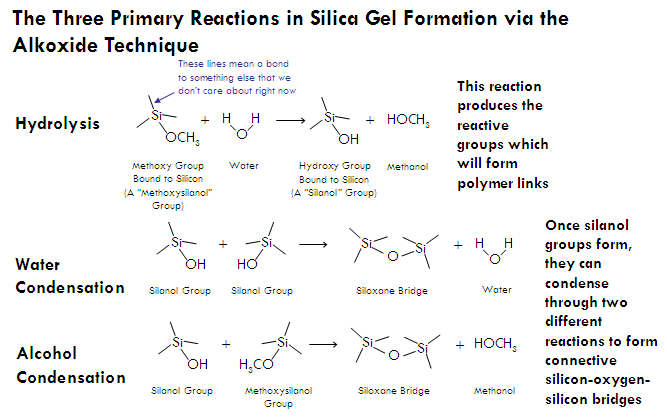
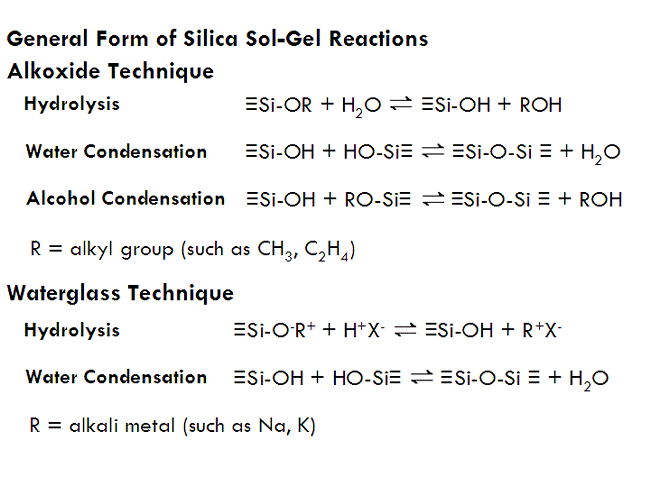
Three reactions result when an alkoxide reacts with water, together resulting in the formation of silica nanoparticles (which are what will later interconnect to form a gel). The first of these reactions is hydrolysis, in which a silicon alkoxide reacts with water to form silanol (Si-OH) groups. These silanol groups can then react either with each other or an alkoxide group (Si-OR) to form a siloxane bridge (Si-O-Si), resulting in the joining of two molecules into one larger molecule. Each silicon atom can form up to four siloxane bridges (remember, silicon is tetravalent, meaning that it is most stable forming four chemical bonds), allowing for many small molecules to connect together into giant molecules which contain thousands of silicon-oxygen bridges. These large assemblies of silicon and oxygen bridges are called silica nanoparticles, and have diameters approximately a few nanometers across.
In general, the composition of a these nanoparticles can be expressed by the empirical formula SiO2, but more accurately by the polymer formula (SiO4)n (in that each silicon atom is attached by a single bond to four oxygens which bridge to other silicon atoms). However, during the formation of a silica nanoparticle, not every silicon atom ends up forming four siloxane bridges and instead hangs on to one or more of its hydroxyl (-OH) or alkoxy (-OR) groups (which would have been used to form a siloxane bond). These are called “terminal groups”, and cover the surface of the nanoparticles.
Formation of a Gel from a Sol
At some point, the nanoparticles reach a critical size where they stop growing in size and instead agglomerate with other nanoparticles. This is dependent on a number of factors, such as the pH and concentration of other species in the solution. The terminal hydroxyl and alkoxy groups on the surface of the nanoparticles allow the nanoparticles to interconnect with each other, kind of like atomic Velcro balls.
When enough of the nanoparticles join together that a continuous network spans the liquid solution, a gel has formed. Often times gels are given special names depending on the liquid solution contained within their pores. Since an alcohol of some sort (such as methanol or ethanol) is frequently used for making silica gels, the term “silica alcogel” is often used. A “hydrogel” on the other hand would contain water in its pores.
Depending on the chemistry of the reaction solution, gelation may just happen immediately following sol formation, or may require additional catalyst, water, temperature, or time to occur. It is also possible that addition of these things will cause the nanoparticles to precipitate forming a white slush instead of a gel. As a result, the chemistry of the system can be quite sensitive.
Throughout the aerogel and, in particular, on the surface of the struts which make up the aerogel’s sponge-like framework, are functional groups where a silicon atom does not share a siloxane bridge with another silicon atom (that is, one of its four bonds does not participate in network bonding). The vast majority of these functional groups are hydroxyl (-OH) groups left over from hydrolysis of the alkoxide, and less so, alkoxy groups which were never hydrolyzed, or reformed after hydrolysis (-OR, such as methoxy).
Drawbacks of the Alkoxide Method
Although the chemistry of silicon alkoxide gelation is very straightforward and elegant from a chemical perspective, alkoxides have their drawbacks. First, alkoxides of silicon as well as alkoxides of metals are hazardous since inhaling their vapors will cause hydrolysis and thus nanoparticle formation to occur in wet tissues such as lungs and nasal membranes. Second, alkoxides tend to be very expensive since they require several chemical steps to prepare. In the case of silicon alkoxides, tetraethoxysilane is often preferred over tetramethoxysilane since it is about four times less expensive (USD$30 per liter vs. USD$120 per liter) and, although less reactive, is also less hazardous (but should still be used in a vent hood!)
But there’s more than one way to make a silica gel though!
Waterglass Gelation: A Cheaper Alternative to Alkoxides
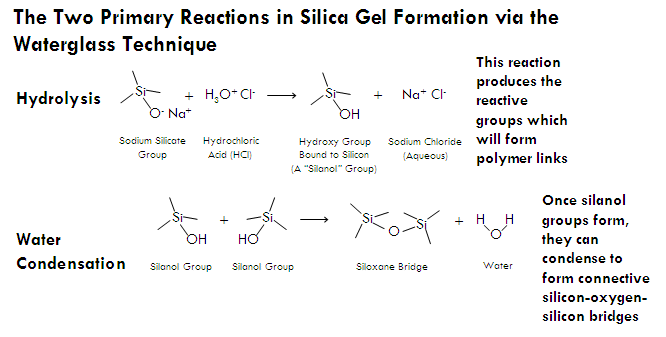
Silica gels can also be produced through a less expensive method involving an aqueous solution of sodium silicate. Sodium silicate is an inexpensive white solid with a range of possible stoichiometries (which each have different names)—Na2SiO3 or sodium metasilicate, sodium polysilicate or (Na2SiO3)n, sodium orthosilicate Na4SiO4, and others. Sodium silicates are soluble in water, and when dissolved the resulting solution is referred to as waterglass or liquid glass.
Unlike silicon alkoxides, sodium silicate molecules do not hydrolyze and condense together when placed in water. However, sodium silicates are slightly basic, and when neutralized with acid (such as hydrochloric or sulfuric acid), hydrolysis will occur and silanol (Si-OH) groups will form. Once silanol groups form, the silicate molecules form siloxane bonds with other silicate molecules and bridge together to form nanoparticles, resulting in a sol. This sol can then be used to make a gel, just as in silicon alkoxide techniques. In the waterglass technique, however, the sol sometimes needs to be heated to 50°C or so to get gelation to occur in short period of time.
Drawbacks of the Waterglass Technique
The waterglass technique may be less expensive and easier than the alkoxide pathway, but the resulting gels are often very fragile and require purification before they can be used to make aerogels (see Purifying and Aging Silica Gels below). Nonetheless, the significantly lower cost of this technique has driven most of the commercial producers of aerogel materials to use waterglass.
What Silica Gels Look and Feel Like
Well-made silica gels are generally transparent exhibiting no color and little scattering effect. Dense gels (0.1-0.5 g cm-3) can be very hard and rubbery—tapping a beaker filled with this density of gel one can feel elastic vibrations resonate in hand. Lower density (0.01-0.1 g cm-3) gels are more like gelatin dessert in consistency, but don’t bend as well. Ultralow density gels (0.001-0.01 g cm-3) are very delicate and do not hold up well under their own weight without a mold. All silica gels tend to fracture or crumble when poked or cut, much more so than gelatin dessert.
Poorly made silica gels will be opaque white or opaque-ish (not totally transparent), and depending on how poorly made they are, slushy with poor monolithicity—more like a slurry than a gel. These properties result from overhydrolysis of reactants or nanoparticles, which causes them to fall out of solution instead of joining up with other particles to form a gel framework. Gelation can also occur unevenly across a container and part of a gel will be well-formed while another part is slushy or even liquid still.
Purifying and Aging Silica Gels
Once a silica gel has been formed, the liquid within the pores of the gel (or the “gel liquor”) usually contains lots of stuff other than just solvent. Gels produced through the alkoxide technique will contain alcohol, water, catalyst, and other random organic compounds. Gels produced through waterglass gelation contain sodium ions, acid, and lots of water.
If the gels are to be supercritically dried to produce aerogels (see How Aerogel is Made), these miscellaneous components (especially water and sodium salts) are not supercritically extracted as easily as the solvent being extracted (CO2, ethanol, etc.), meaning they’ll be stuck in the pores of the resulting aerogel. This can cause reduced transparency and mechanical strength, shrinkage and/or cracking of the monolith, and off-gassing and/or residual wetness after the aerogel is made. Impurities are more significant in Hunt Process (CO2) drying than in direct supercritical extraction of an organic solvent (such as methanol or ethanol) since CO2 is very non-polar, and doesn’t dissolve water or sodium salts well even in its supercritical state.
As a result, the gel liquor must be purified. This is done by simply soaking the gel in a pure solvent, usually an organic solvent such as methanol, ethanol, or acetone. As the gel soaks in the pure solvent, impurities will diffuse out and pure solvent will diffuse in until an equilibrium concentration of species is reached. Depending on the size and shape of the gel, this can take anywhere from hours to days. The process is repeated with fresh solvent a number of times to ensure adequate removal of impurities from the gel.
During this purification process, some final chemical reactions which contribute to the strengthening of the gel’s structural framework may occur as well. This is referred to as aging.
Once the gel is purified it can be dried through supercritical drying or subcritical drying to produce silica aerogel.
Naming of Gels
Often times you will see gels referred to as “alcogels” or “hydrogels”. These names refer to a gel with a specific gel liquor, that is, liquid contained within the pores of the gel. So:
- Alcogel = gel with alcohol in its pores
- Hydrogel = gel with water in its pores
You will usually see the term “silica alcogel” used in reference to gels prepared through the alkoxide technique, since alcohol is usually the solvent, and “silica hydrogel” for gels prepared through the waterglass technique, since the solvent is usually water.
But because you can easily exchange the liquid in a gel with another liquid by soaking, and because every time you do a solvent exchange you technically would have to rename your gels (for example, “ketogels” if you solvent exchange into acetone, “carbonogels” if you exchange into liquid carbon dioxide), on Aerogel.org we just call every wet gel a “gel”. Just remember gels are NOT aerogels–they are the precursors to aerogels. And remember that “silica gel” is often used in industry to refer to silica xerogel.
Structure of a Silica Aerogel
The solid framework which makes up a silica aerogel is composed of nanoparticles of silica—the oxide of silicon—just like glass, quartz, or sand (although these forms of silica often have other components mixed in). The way these nanoparticles connect together to form the framework of the aerogel can vary, and is generally a result of the chemistry used to prepare the aerogel precursor gel. In an silica aerogel produced through a typical base-catalyzed alkoxide sol-gel process, nano-sized primary particles of silica 2-50 nm in diameter are agglomerated into spherical secondary particles 50 nm-2 um (2000 nm) in diameter which are then in turn connected together in strands in a “string of pearls” type structure, which you would see if you were to look at the aerogel under a scanning electron microscope (SEM). In silica aerogels produced through an acid-catalyzed sol-gel process, the smaller primary particles do not agglomerate into larger spherical secondary particles and are instead connected in a leaf-like morphology.
What a Silica Aerogel Looks and Feels Like
A high-purity, well-made silica aerogel will be highly transparent exhibiting a characteristic blue cast caused by Rayleigh scattering of the short wavelengths of light off of nanoparticles which make up the aerogel’s framework. To the touch it may feel sticky, with various consistencies depending on the density of the aerogel. Here is a descriptive guide to help you imagine touching a silica aerogel:
- Low Density (0.001-0.01 g cm-3): Spongy, in between a StyrofoamTM peanut and green floral potting foam but not squishy and instead fracturing when squeezed
- Medium Density (0.01-0.1 g cm-3): Similar to above, but with a mechanical strength and consistency between a piece rigid StyrofoamTM and CheerioTM or a Rice KrispieTM.
- High Density (0.1-0.5+ g cm-3): Almost exactly like astronaut (freeze-dried) ice-cream in consistency but dry, breaking it reminds you of snapping a piece of glass or acrylic, but much easier
Silica aerogels feel sticky because of the unreacted silanol (Si-OH) groups which terminate the skin of their skeletons—the -OH groups make weak hydrogen bonds easily, not unlike ScotchTM tape does, which makes it feel sticky.
Silica Aerogels and Water
As mentioned above, silica aerogels usually contain lots of unreacted silanol (Si-OH) groups on the surface of their skeletons. The presence of these groups makes silica aerogels very hydrophilic—that is, they readily soak up water upon contact and from vapor in the air.
When an aerogel soaks up water, the water nestles into nanopores in the aerogel’s skeleton. This can cause the aerogel to become cloudy (lose transparency), and even shrink and/or crack. An initially transparent silica aerogel sitting on a table may become cloudy over the course of months to years because of water vapor working its way in. Touching a silica aerogel may leave a white fingerprint behind. Dunking a silica aerogel under water generally causes the monolith to rapidly (and irreversibly) shrivel up. This applies to other polar solvents as well. On the flip side, silica aerogels repel non-polar liquids such as benzene, hexane, and gasoline very effectively.
Silica aerogels dried by Hunt Process drying are generally very hydrophilic, unless special steps have been taken to water-proof them as described below. Silica aerogels dried by high-temperature supercritical extraction of methanol, on the other hand, are usually reasonably hydrophobic (repel water). This is because at high temperatures methanol reacts with surface silanol groups to form methoxysilanol groups (that is, Si-OH becomes Si-OCH3). Water doesn’t stick to methoxy groups as well as it does to hydroxyl groups since they are less polar than hydroxyl groups. As a result, water and other polar solvents are not drawn into the aerogel as easily.
Water-Proofing and Functionalizing Silica Aerogels (The Fun Stuff!)
Silica aerogels are particularly easy to functionalize—that is, attach chemicals to the surface of their skeletons—because of all the silanol groups (Si-OH) on their surfaces. Think of these silanol groups as being molecular electrical outlets that we can plug useful molecular appliances into! During the formation or purification steps of silica gel production (and even during supercritical drying), chemicals can be introduced into the pores of the gel which will react with the gel’s silanol groups, resulting in the attachment of other chemical groups to the gel’s surface. When the gel is dried to make an aerogel, these groups stick around and coat the surface of the skeleton of the resulting aerogel.
This is where chemists and materials scientists get to have some fun! You could replace the silanol groups with a non-polar group, such as trimethylsilyl -Si(CH3)3, which would make the resulting silica aerogel water-proof! Or you could add a fluorophore, which would make the resulting silica aerogel glow under a UV lamp! All sorts of neat things can be attached to silica aerogels to make useful aerogel materials. One interesting example is the attachment of molecules which fluoresce when exposed to oxygen, resulting in silica aerogel that can act like an oxygen sensor!
Water-proofing aerogels is useful especially when using silica aerogels in an application where they need to be transparent, such as in window insulation or a Cherenkov radiation detector. One chemical used to water-proof silica aerogels is hexamethyldisilazane, a compound used to water-proof other materials, which plants those trimethylsilyl groups mentioned earlier over the surface of the gel.
Read more about functionalized aerogels under Functionalization of Aerogels.
Materials Properties of Silica Aerogels
Silica aerogels are best known for combining high optical transparency with tremendously low thermal conductivity, which can take on values as low as 0.015 W m-1 K-1. That’s something like the equivalent insulating value of a stack of 30 panes of window glass compacted into a single inch (2.54 cm)!
See how silica aerogels size up against other types of aerogels and other materials on the Properties of Aerogels page.
Uses of Silica Aerogels
Silica aerogels have found a wide array of uses, mostly in high-tech science and engineering, but are beginning to enter the commercial mainstream. Below are a few examples of where silica aerogels have helped out.
Insulation on the Mars Exploration Rovers
The extreme temperatures of Mars, which can range from -220°F (-140°C) to 70°F (+20°C), can result in some pretty serious temperature gradients and thermally-induced stresses in sensitive electronics. Thanks in part to the impressive insulating abilities of silica aerogel, the electronics on the twin Mars Exploration Rovers, Spirit and Opportunity, have managed quite nicely on the Red Planet and have well outlasted their design expectations.
Hypervelocity Particle Capture in the Stardust Probe
Because of its intricate nanostructure and cellular structure, aerogels are excellent at energy damping—and not only just thermal energy damping but also acoustic, electrical, and impact damping as well. So when Dr. Peter Tsou of NASA’s Jet Propulsion Laboratory sought out to collect a sample of comet dust to understand the origins of our Solar System, he looked to silica aerogel as a way to catch it. Comets eject a plume of particles and dust which give them their characteristic luminescent tail (or coma). Tsou headed a project called Stardust designed to collect ejected particles from comet Wild 2. Relative to the probe, the impact velocity of the particles ejected from the coma was approximately 6100 m s-1 (13,600 mph). Albeit the particles being ejected were on the order of a grain of sand in size, at those velocities such particles pack quite a punch! Using blocks of a special three-layer gradient density silica aerogel, the Stardust probe was able to safely and softly cushion the impact of these particles to a complete stop, leaving the particles embedded in the aerogel collector for extraction and analysis upon returning to Earth.
Read more about the Stardust Mission.
High-Energy Cherenkov Radiation Particle Counters
In the sci-fi series Star Trek, when a body (such as the Starship Enterprise) jumps to warp speed, it exceeds the speed of light and releases a brilliant flash of light in what amounts to the optical analog of a sonic boom. It turns out that this can actually happen
(to some extent). The speed at which light travels through a material is different depending on the material, and in some cases, is so low that particles with mass (such as an electron) can actually move faster through the material than light can. When this happens, a cone-shaped electromagnetic shockwave of light is emitted along the trajectory of the moving particle, manifested as a sort of an ectoplasmic blue glow. This effect is called Cherenkov radiation, and it is a way to detect high-energy particles in a particle reactor. Because of its nanostructured pore network, the speed of light through low-density aerogels is very low, and, in the case of a silica aerogel, the material’s transparency allows for viewing of the glowing Cherenkeov light. Aerogels have been used at DESY, Germany’s national particle accelerator laboratory in Hamburg, and CERN in Geneva, Switzerland as Cherenkov radiation detectors.
Remediating Oil from Water
Hydrophobic silica aerogels aren’t just good at repelling water, but are actually also remarkably good at absorbing other substances which repel water—substances like oil, for example. As a result, specially-functionalized silica aerogel powder and blankets have been proposed for use in oil-spill cleanups, and are being developed commercially for this application.
Transparent Window Insulation?
One of the tantalizing commercial applications of silica aerogel is for use as insulation in transparent windows. In terms of heat management, windows are the lossiest materials in pretty much any building (although notably more heat is lost through walls and ceilings than through windows since they represent so much more surface area). Combining optical transparency up to 99% and the thermal conductivities as low as 0.02 W m-1 K-1, it seems like this would be an obvious application for silica aerogel, however due to the complexity in producing monolithic windowpane-sized pieces of silica aerogel, this is an area which likely will take another decade or two to reach consumers.
Airglass AB of Sweden has made windows of silica aerogel on a very limited basis. In fact, their workshop windows are insulated with silica aerogel!
Cabot Corporation licenses a technology to produce translucent granules of silica ambigel (ambiently-dried aerogel). Their product, Nanogel®, comes as a loose mixture of powder and small sub-centimeter-sized particles which have decent insulating ability and around 60% optical translucency. This material has been used in diffuse-lighting skylights in buildings.
Thermal Insulation Without the CFCs
One of the most enticing applications for silica aerogel is for use as ordinary, non-transparent insulation. Aspen Aerogels produces a family of products based on silica aerogel which has been formed around a mesh of some sort and then supercritically dried, resulting in a flexible (but somewhat crumbly) opaque aerogel mat. Different formulations of these materials are designed for use in cryogenics, high-temperature systems, and even in winter clothing. A major application of Aspen’s silica aerogel blankets has been in insulating subsea oil pipelines which require a layer of insulation to keep viscous, slag oil warm enough to keep flowing—since a much thinner layer of aerogel blanket can be used to do the job of a thicker layer of traditional fiberglass or mineral wool insulation, a subsea oil pipeline insulated with aerogel blankets can be made much thinner. This translates to greatly reduced costs when laying oil pipelines, since the smaller pipes can be laid down by a great many more number of ships than the traditional larger pipelines.
A major advantage to silica aerogel as a thermal insulation is enhanced energy efficiency and, in turn, reduced harmful emissions resulting from energy consumption. Energy-intensive refineries, for example, predominantly use mineral wool as an insulating medium. Silica aerogel materials are poised to displace mineral wool in the decade, translating into greater efficiency and reduced emissions in what amounts to 15% of all industrial CO2 emissions in the United States. Furthermore, unlike polyurethane and polystyrene insulations, no chlorofluorocarbon (CFC) blowing agents are required. In fact, Aspen Aerogels maintain closed-looped supercritical drying systems in producing silica aerogel, meaning no carbon dioxide from supercritical drying is released as a result of its manufacture.
The Future of Silica Aerogels
From a nanotechnology perspective, silica aerogels will continue to serve as the staple substrate for scientists seeking to develop nanotechnologies which rely on high surface areas or nanoporosity. Things like chemical and biological sensors will benefit from silica aerogel-based technologies in the coming decade.
As far as insulation is concerned, the future of silica aerogel may be very well lie in already-developed technologies. Both transparent and opaque silica aerogel-based insulation has tremendous potential to displace technologies such as mineral wool, fiberglass, foams like Styrofoam® and polyurethane in the future, even thermalpane windows, but only once their production has reached a level of efficiency and cost competitiveness with existing technologies. That time is not far away (possibly 2015-2020), and companies like Aspen Aerogels have already started paving the way towards it.