Part 4: The Early Days of Aerogels
The Enigmatic Discovery of Our Favorite Material
By Dr. Mike Ayers, Lawrence Berkeley National Laboratory
Aerogel.org Contributor
May 2000
The most fascinating of Samuel Kistler’s many discoveries must surely be the one that he made the earliest in his career-namely, the formation of the world’s first aerogel. He accomplished this by the now classic process of supercritical fluid drying, a relatively simple feat today, but a much more daunting task in the days when pressure vessels were not off-the-shelf items.
The First Aerogels
However, it is not completely clear exactly when and where this discovery was made. Kistler had a keen interest in supercritical fluids from the beginning of his education. His Master’s thesis, which he completed at Stanford in 1922, proposed the crystallization of amino acids from supercritical fluids. Kistler wrote the following in its introduction:
In the fall of 1920, I became interested in some research work on the crystallization of amino acids from solution carried on by Dr. E. L. Alling, then at Stanford University, and during the ensuing winter and spring I became closely associated with him and his work. The present investigation is an outgrowth of the problems we were confronted with during the year.
The standard method of analysis of proteins is to hydrolyze them to amino acids, esterify the acids, and separate by fractional distillation. This method is long and tedious, and at best accounts for only from 85-90% of the protein. A careful analysis involves such time, as much as four months, that the chemist hesitates to employ it and rather resorts to other methods of distinguishing proteins and determining their properties. Dr. Alling hoped to discover a simpler method of the amino acids present in the hydrolized protein, as well as to obtain greater accuracy, by the application of the petrographic microscope…
…It occurred to Dr. Alling that if a solution of amino acids, in some solvent whose critical temperature was not so high as to decompose them, were gradually brought into the neighborhood of the critical temperature, crystallization might be induced since the solubility decreases rapidly as that temperature is approached, and the log of the viscosity decreases inversely as the log of the temperature…I therefore proposed to investigate the precipitation of compounds from solution as the critical point is approached, paying special attention to indications of supersaturation. I have made a careful study of the literature and at my earliest opportunity intend to conduct experiments in the laboratory designed to demonstrate the feasibility of this method of crystallization…
There is no record available as to whether he ever got around to conducting his experiments, but as he shortly became employed by Standard Oil this does not seem likely.
As mentioned in the previous section, Kistler spent the years of 1923-1930 teaching undergraduate courses at the College of the Pacific in Stockton, California, a school with limited facilities at a newly constructed campus. The first paper on aerogels was published in Nature in 1931 (vol. 127, p. 741), so the work on aerogel must have been performed near the end of his employment at Pacific. However, Kistler began his doctoral work at Stanford during the summer of 1927 and continued his work there in subsequent summers. It could logically be assumed, therefore, that the first aerogels were made during these summers at Stanford, where facilities were more readily available.
This does not appear to be the case, however. There is no doctoral dissertation on record at Stanford for a S. Kistler, nor is there a reference to one in Dissertation Abstracts International. It is not clear, then, exactly what Kistler’s doctoral work involved. What is known is that he worked closely with Professor J.W. McBain, publishing more than one paper in the late 1920’s concerning the use of wet gels as ultra-filters. It is probable that this work was his major doctoral research. Clearly it was his first experience working with gels in general.
A revealing clue is found in Kistler’s detailed publication of the first aerogels in the Journal of Physical Chemistry (vol. 36, p. 52, 1932) where he wrote the following acknowledgement:
“In conclusion, I would like to express my gratitude to Mr. Charles H. Learned for long hours of patient labor in the laboratory, and also to Dr. J. W. McBain for the loan of apparatus and for kindly assistance and advice.”
The registrar’s office at Pacific has confirmed that Charles H. Learned was enrolled as an undergraduate in Stockton in the late 1920’s, and this, combined with the fact that McBain loaned Kistler equipment, makes it highly likely that the first aerogels were indeed created in Stockton, at the College of the Pacific.
One final item remains a mystery, namely the origin of the word “aerogel” itself. There is no record as to whether Kistler himself first coined the term, whether he heard it from someone else, or whether he had considered any other names for the substance (frozen smoke, perhaps?) This will likely remain an unknown part of aerogel lore in perpetuity.
Work at Illinois
During the four years that Kistler spent at the University of Illinois (1931-1935), aerogels appeared to be the focus of his research efforts. Many important early publications on the properties of aerogels came out of his work there, including studies on the thermal conductivity of silica aerogel, and the catalytic properties of various oxide aerogels. These topics continue to fascinate researchers to this day.
The First Commercial Aerogel
Samuel Kistler never seemed to pass up an opportunity to commercialize his inventions, and aerogels were no exception. In the early 1940’s he completed a license agreement with the Monsanto Corp. for the production of silica aerogel. Monsanto began production in a plant in Everett, Massachusetts, and sold products for many years under the trade names “Santocel”, “Santocel-C”, “Santocel-54”, and “Santocel-Z”.
As Monsanto moved from pilot plant operations to full-scale production, a paper was presented at an ASME meeting by Monsanto researcher John White (Trans. Am. Soc. Mech. Eng. 1942 p 435):
MANUFACTURE OF SILICA AEROGEL-DESCRIPTION OF PROCESS AND HEAT TRANSFER PROBLEMS
BY JOHN F. WHITE (Non-Member)
Presented at the Joint Symposium with the heat Transfer Division of the American Society of Mechanical Engineers at the Boston, Massachusetts, Meeting, May 13, 1942
ABSTRACT The basic theory underlying the method of producing aerogels and a general description of the process are given. Particular attention is given to the heat transfer problem, involving the heating of a SiO2 alcogel to temperatures and pressures above the critical points for the liquid phase present in the gel. It is shown that the major portion of heat transfer is accomplished by convection, the amount being transferred by radiation and conduction being negligible in the pilot plant autoclave described. The shapes of the isotherms existing in the autoclave and their rate of travel have been given, supplying data useful for design purposes. The advantages of condensing diphenyl vapor for supplying heat at high levels to pressure vessels have been discussed.
The paper continued to describe the thermal cycle encountered in the plants large high-temperature autoclaves. At the end of the talk the customary question and answer session ensued which was recorded and published with the proceedings. The only questions came from none other than one Dr. S. S. Kistler:
S. S. Kistler (The Norton company, Worcester, Mass.): I should like to ask Mr. White what percentage of free space occurs in the dry gel in the autoclave.
J. F. White: By that I presume you mean the dry gel as charged to the autoclave. That will range about 30 per cent void space.
S. S. Kistler: The reason I asked that question was because of the peculiarity of the heat transfer inside. If alcohol vaporizes from the surface of the autoclave, it should condense on the interior, but I noticed that the temperature curves for the second and third thermocouples don’t start to rise for about twenty-five to thirty-five minutes after the heat is on, whereas the vapor pres- sure is adequate to raise the temperature very rapidly. I wonder if we could have the second slide, the slide showing the curves of heat in the autoclave. (Slide): You notice the broken line on the side shows the equilibrium temperature, the saturation temperature. The bottom, second and third thermocouples don’t begin to show a temperature rise up to half an hour after the diphenyl valve is opened, which looks as though there is an air lock inside that prevents penetration of the alcohol vapor to the interior of the mass.
J. F. White: It would seem rather difficult to understand how the air could be locked in this mass. I would assume the air to come out almost at once under the conditions there; that is, I rather question that there is an air lock, although I really cannot be sure of that.
S. S. Kistler: The temperature throughout the mass should be uniform as long as the vapor is not blocked by either air or liquid, and if you have 30 per cent free space, in order to block that space with liquid, it means condensation of 30 per cent of alcohol, which should raise the temperature quite materially.
J. F. White: I can show that it is not only an air lock because if I had put on curves for a batch in which all the space had been filled with alcohol prior to the application of heat so that there would be no possibility of air in the autoclave, we would have found a similarly shaped curve, except that curves 2 and 3 would be horizontal for a much longer time. As a matter of fact, they would not start to rise for a matter of an hour or an hour and a half after the application of the heat, and during that time we would be driving off alcohol at 600 degrees Fahrenheit, or somewhere within that range, at 1100 pounds of pressure.
S. S. Kistler: In that case I suspect that you would have reduction in the heat transfer due to low conductivity of the alcohol itself; whereas, here you have an opportunity for vapor penetration, which is the best method of heating available. Have you ever tried evacuating the autoclave before turning on heat?
J. F. White: No, that has never been done.
S. S. Kistler: Perhaps you could explain the crook in the curve.
J. F. White: No, I cannot explain the crook in the curve, but I intentionally put it in. It could have been left out and a much smoother curve shown. But, as a matter of fact, with mechanically recorded temperature curves in about 10 per cent of all our batches we find a dip such as that denoted in curve No. 2. It is difficult to explain. There is some shrinkage during the auto-claving. It amounts to about 5, or perhaps 10, per cent of the total volume, and the mess loosely sinters together in there. It is a very loose sintering. I sometimes wonder if there is not a drawing together of the mass and then perhaps sort of a bridging or arching effect occurring there and then suddenly that arch breaks and the whole mass drops downward. But, as a matter of fact, if it dropped back under a bit, you should get a jump rather than a drop. However, I cannot see any good reason for a drop like that, but I put it in there, hoping that someone would have the answer.
The Monsanto Aerogel Plant
The following description of the Monsanto aerogel plant appeared in Chemical and Metallurgical Engineering February 1943 p.144. How would today’s Environmental and Health standards have affected this plant? No one can say.
Production of Silica Aerogel
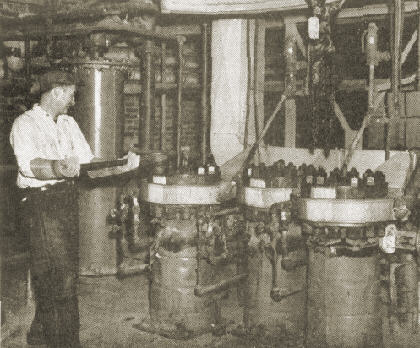
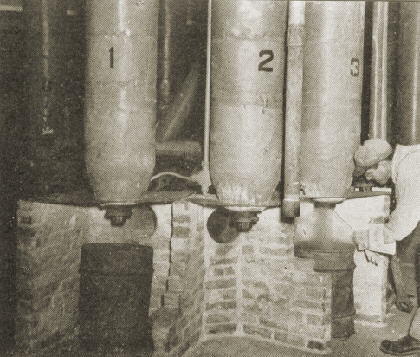
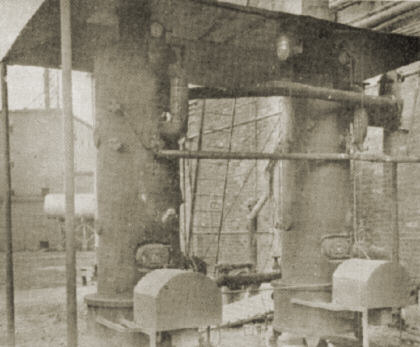
The first large scale production unit for silica aerogel has recently commenced operations at the plant of Monsanto Chemical Co. at Boston, Mass. The product is a light, friable, slightly opalescent solid containing as much as 95 percent air volume. It is a very effective heat insulating material. Silica aerogels having densities as low as 1.8 lb. per cu.ft. have been produced.
The process consists of adding a solution of sodium silicate to sulphuric acid. Concentrations are controlled to yield a gel having 8 percent silica. After aging several hours to allow the gel to strengthen, due to syneresis phenomena, it is removed from the tank and passed through the roll crusher into one of four wash tanks. Water is passed up through this tank over the gel to remove the sodium sulphate formed in the gel preparation reaction. When the gel has been sufficiently washed, all excess water is removed by draining and the gel is then covered with alcohol. After a suitable soaking time the alcohol is drained off and replaced with a fresh portion. The alcohol washing procedure is done by the use of a conventional 4-stage counter-current system. Greater economy of alcohol is realized by using the cover, soak and drain method of washing in place of continuous how through the tanks. The counter-current cycle is facilitated by use of the wash receivers and the transfer pump. Weak alcohol taken from the system is recovered as strong alcohol by fractionation in a recovery column.
When the water in the gel has been substantially replaced with alcohol, the excess alcohol is drained off and the gel charged to the autoclave. Here it is heated to 550 deg. F., the pressure being held to 1150 lb. gage by bleeding of alcohol vapor through the condenser. When 550 deg. F. is reached the pressure is reduced to atmospheric and the autoclave is finally evacuated to 20 in. mercury for 10 min. The resultant aerogel is then removed by a conveyor system. The autoclave is heated by a jacket containing diphenyl vapor at 80 lb. gage pressure, this vapor being supplied by the oil fired diphenyl boiler.
The process is built around one essential unit operation involving a unique step, i.e., the heating of a gel system to temperatures and pressures above the critical for the liquid phase present in the gel.
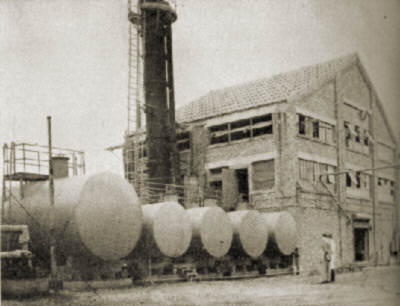
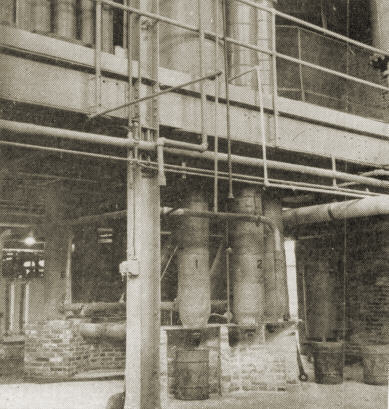
1. First large-scale production unit for silica aerogel has been completed at Boston. Alcohol storage tanks in foreground.
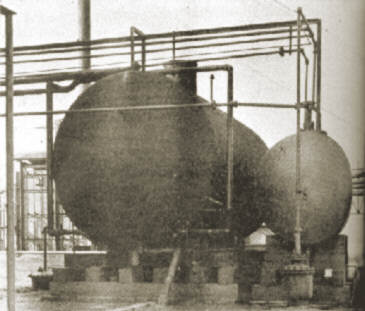
2. The process consists of adding a solution of sodium silicate to sulphuric acid. Storage tanks for these materials are shown here.
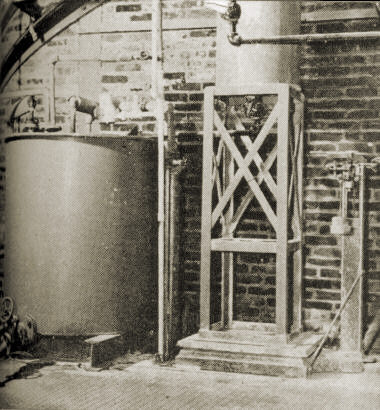
3. Acid weigh tank and dilution tank used in controlling concentrations to yield a gel having 8 percent silica.
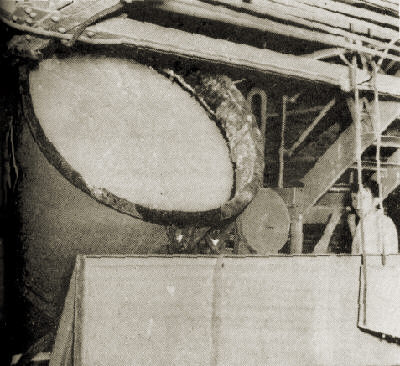
4. After several hours it is removed from the tank through a roll crusher into one of the four wash tanks.
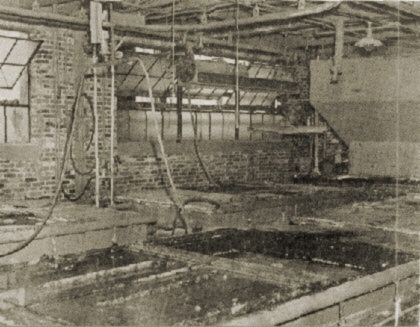
5. Water is passed up through these wash tanks over the gel to remove sodium sulphate formed. Traveling roll hopper in background.
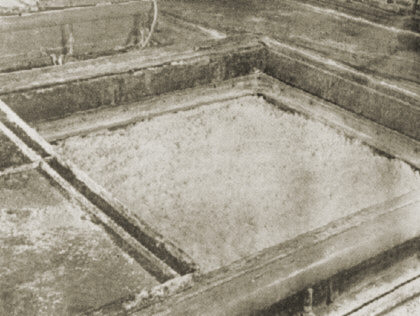
6. The alcohol washing procedure is carried out in a different system. The excess alcohol has been removed.
See a schematic diagram of the Monsanto aerogel plant.
Who Bought This Stuff Anyway?
In those days Monsanto apparently did not possess the savvy marketing expertise that the company does today, and there is surprisingly little information about the Santocel line of products in relevant trade publications or other literature of the time. A few tidbits of interest can be found in Monsatno Annual Reports to Shareholders:
The Monsanto Annual Report (1948) had the following photo:

Publicity picture for Santocel, a Monsanto product based on aerogels, shown in Monsanto Annual Report
and the 1951 Annual Report mentioned:
“Significant and unusual applications for Santocel, outside the flatting and insulation fields, were developed for civilian and military use. Among these were the Department of Agriculture’s approval of Santocel as a thickening agent for screwworm salves for sheep, and its use as a thickening agent in the jelly of the fiery Napalm bomb. Santocel also has become an essential ingredient in the manufacture of silicone rubber.”
Napalm and cigarettes! Nice going Monsanto, it is no wonder this product never went anywhere! In reality it seems that the major application for Santocel at the time was, in fact, as a flatting agent in paints and varnishes. A product report in the Paint Industry Magazine (November, 17, 1947) gives the following description of this application:
Santocel –A New Raw Material for the Coating Industry
By Chester L. Jones, Monsanto Chemical Co, Everett, Mass.
Thursday, Nov. 13, 1946
Many new raw materials have been made available to the coating industry in the last few years. These have included film-formers, solvents, and pigments. I have been asked to speak about one of them, Santocel, an inert pigment having unique properties.
Santocel is not new to your technical men. It was produced in small quantities before the war. During the war it was used in some special products but it was not possible to supply the demand, or expand production to the point where it could be offered generally. A new plant has been erected during the past year, making it possible to supply the product for all uses.
Santocel is silica in a relatively pure state, being about 90 percent SiO2 and the balance, volatile matter with a small percentage of sodium sulfate. The unique property of this material is due to its form and not to its composition. Technically, it is called an Aerogel because of its structure.
The immediate reaction of a person in the paint industry upon learning that Santocel is silica is that it is low priced, and used as an inert filler or extender. Neither assumption is correct. It is not cheap, and it can not be used as an extender.
To adequately describe the product requires a brief outline of the method of production. Essentially the process of manufacture of Santocel consists of adding dilute sodium silicate to dilute sulfuric acid. In time the mass will form a solid gel, even with very low silica content. Sodium sulfate is removed and water displaced with alcohol. The gel is heated in an autoclave under pressure until critical conditions are reached at which time the liquid is instantly converted to a gas and is released. Such a process results in no shrinkage of the gel. The only change is that air has replaced the liquid and the gel is dry. The product resembles the wet gel in structure, thus the term Aerogel.
The air volume of Santocel is 94 per cent. It males no difference how fine you break down the particle, it still has the 94 per cent air volume in its cells. Thus it is exceedingly light in weight and exposes a tremendous surface of silica. The internal surface of the particle is exposed as well as the external. Santocel has been compared to anything from white lampblack to powdered air. The dry bulk density of this material as it is supplied to the industry is approximately six pounds per cubic foot. However, when the air in it is displaced by a liquid, the bulking or absolute density is the same as for my other silica.
The question is immediately asked-what effect does this sponge-like structure have on the properties of silica, when used in coatings, that is different from any other form of silica? The most apparent visual effect is that Santocel imparts very little or no opacity to the vehicle, this is easily explained when we visualize the 94 per cent air volume being displaced with the vehicle. The cell walls are so thin that they offer little opacity and since silica has an index of refraction not too different from most coating vehicles, it is not surprising that it does possess such a low degree of opacity. A second visual observation is that the product produces a marked thickening effect on most vehicles to which it is added as compared to other pigments. It is for this reason that it cannot be used as an extender. It is rather difficult to incorporate quantities in excess of 15 per cent even in grinds. It has a higher oil absorption than any common pigment.
A material with these characteristics immediately suggests itself as a flatting agent and work to date has shown that it excels in every instance where it can be used successfully. As a flatting agent, it is more efficient on a weight basis than any other material used for this purpose. It is not surprising that this is so when we analyze the effect of its structure….
One other item of interest can be found in a Monsanto Annual Report from the 1940’s:
“Santocel, a unique form of silica, is a flatting agent in coatings and also a thermal insulating agent. The Everett plant also produces alcohol, dry ice, and ethyl acetate.”
One immediately wonders whether the presence of alcohol and dry ice in the same manufacturing plant was a curious coincidence, or if the scientists at Monsanto had managed to anticipate the future discoveries of TEOS gels, and supercritical carbon dioxide drying?
In any case, Monsanto abandoned Santocel sometime around 1970, most likely due to its high manufacturing costs and competition from other types of silica and thermal insulations.