Nanotubes and Aerogels
This article describes the intersection of two intriguing materials–aerogels and nanotubes.
There are a couple different ways nanotubes and aerogels have come together:
- Aerogels can be made out of carbon nanotubes
- Carbon aerogels can be reinforced with embedded carbon nanotubes
- Metal oxide aerogels can be used as catalysts to grow carbon nanotubes
- Metal-doped carbon aerogels can act as catalysts for growing carbon nanotubes
- and, lastly, fluffy masses of nanotubes formed during nanotube yarn production and sheets of entangled carbon nanotubes are sometimes incorrectly referred to as aerogels
If you’re new the nanotube scene, here’s some background on what is one of the most prominent areas of nanotechnology. Otherwise skip down below to read about aerogels made with carbon nanotubes and nanotube catalysts made out of aerogels.
What are Nanotubes?
Nanotubes are nanosized structures which nanotechnologists can use to make electronics, sensors, drug delivery vehicles, and materials. A nanotube is a hollow, high aspect ratio (that is, much taller than it is wide) cylindrical structure with a diameter of <100 nm. The term nanotube refers to a geometry a substance can take on and not to a substance itself. Nanotubes of metals, metal oxides, metal silicides, metal nitrides, polymers, silicon, and carbon have all been synthesized; however, the synthetic routes for preparing nanotubes vary greatly depending on the desired composition of the nanotube.
Of the different types of nanotubes that have been developed, by far the most important and most heavily researched type is the carbon nanotube (CNT). In fact, just as many people use the term aerogel to refer specifically to silica aerogel, the term nanotube by itself is frequently used to refer specifically to carbon nanotubes.
In this section we will talk about the intersection of carbon nanotubes and aerogels.
About Carbon Nanotubes
What are Carbon Nanotubes?
Carbon nanotubes are seamless, hollow cylinders of carbon atoms in which the carbon atoms are arranged in a regular hexagonal array. The structure of a CNT shell is similar to a single layer of graphite (called graphene), namely, a regular, planar array of carbon atoms, each of which is connected to three other carbon atoms in an sp2-hybridized bonding configuration (a hexagonal patterning of the atoms), but with the important difference that a CNT is cylindrical and not flat. Carbon nanotubes can be comprised of a single shell, in which case they are called single-wall carbon nanotubes (SWNTs), or can consist of multiple, concentric shells, in which case they are called multi-wall carbon nanotubes (MWNTs). Because the atoms in a carbon nanotube are covalently bonded in a regular array, nanotubes are effectively crystalline materials. SWNTs are typically around 1 nm in diameter and MWNTs are typically 10-50 nm. Remarkably, carbon nanotubes can be up to several centimeters in length giving them, an aspect ratio of thousands to millions. Because of their incredibly high aspect ratios, unlike other crystalline materials like diamond or quartz (which are brittle), carbon nanotubes are flexible and can be wound into fibers and sheets. Depending on the application, most nanotubes range from about 1 micron to 1 mm in length.
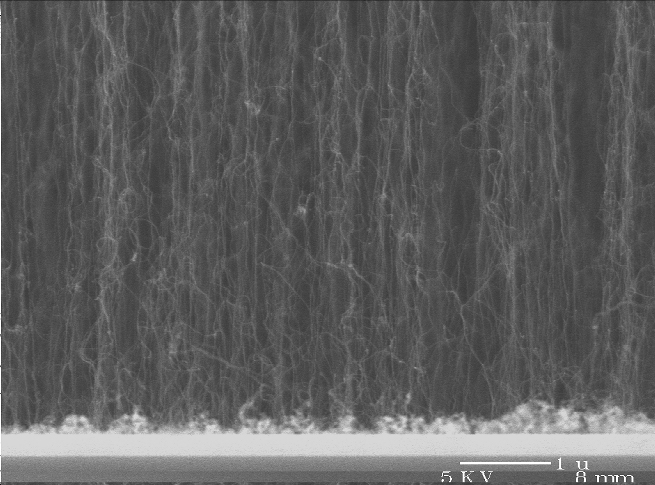
Vertically-aligned multi-wall carbon nanotubes grown from iron nanoparticles on a silicon substrate (image courtesy of Alfonso Reina, MIT).
What is the Diameter of a Carbon Nanotube?
There are a large number, but a finite number, of single-wall carbon nanotube diameters possible, the smallest being 0.393 nm in diameter. Why are there no smaller SWNTs? If you were to take a molecular modeling set and try building a model of a carbon nanotube, you would find that you can only fit together cylinders with certain diameters containing a certain number of carbon atoms per unit of cylinder height. This is because carbon atoms are restricted to making bonds at certain angles and, in a nanotube, each carbon atom (ideally) also has to make exactly three bonds to other carbon atoms. The smallest carbon nanotube is the most the carbon atom bonds can be bent and still result in a structure that is stable (not spontaneously break open).
The smallest multi-walled carbon nanotube is a double-wall carbon nanotube (DWNT), in which one nanotube is nested concentrically in another. Generally the spacing between neighboring nanotubes in a MWNT is as close as possible and around 0.4 nm (similar to the spacing betweens planes of carbon atoms in graphite).
Electrical Conductivity of Carbon Nanotubes
Each different nanotube diameter results in hexagonally-patterned carbon atoms arranged in one of three ways: one in which the hexagons stack up the length of the nanotube flat side facing up; one in which the hexagons stack vertically with a vertex pointing up; and one in which the hexagons spiral up the length of the cylinder like stripes on a candy cane. Depending on which pattern of hexagons that the nanotube exhibits (that is, its chirality), the nanotube will conduct electricity either like a metal or a semiconductor. This is an amazing property of carbon nanotubes, since we can (in principle) make nanostructures with conductivities that resemble metals, like copper, or semiconductors, like silicon, using only carbon atoms arranged in different ways! That means that it may be possible to build integrated circuits (computer chips) completely out of carbon nanotubes. Furthermore, because of their incredibly small size, carbon nanotubes could enable production of much smaller transistors and circuit components than processors commercially available today, meaning faster and more efficient computers.
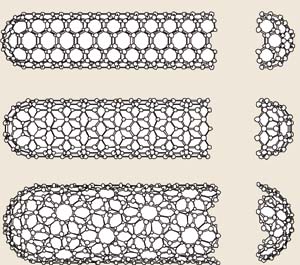
Schematic representations of carbon nanotubes of the three different chiralities--armchair, zigzag, and chiral. The chirality determines whether or not the carbon nanotube conducts electricity like a metal or a semiconductor. (Image credit Physics World)
How Carbon Nanotubes Are Grown
Carbon nanotubes are grown through a couple different techniques but the most popular and versatile technique is chemical vapor deposition (CVD). In CVD, a vapor of a chemical is flowed over a substrate. When the vapor contacts the substrate, a chemical reaction occurs and a material is deposited on the substrate. Usually, but not always, the substrate is hot so that when the vapor contacts the substrate it thermally decomposes into the material you want to deposit. CVD growth of carbon nanotubes is a variation on this technique. Here’s how it works:
- First, the substrate needs to have catalyst nanoparticles where you want the nanotubes to grow. These nanoparticles serve as the “seeds” from which the nanotubes grow. The nanoparticles can be composed of lots of different substances, but usually a metal like Fe, Co, or Ni is used.
- The substrate is then heated to a temperature of ~700°C under H2. As the nanoparticles heat up, the hydrogen pulls oxygen out from the nanoparticles (if they have been oxidized) leaving behind metallic nanoparticles.
- Once at the target temperature, a carbon-containing gas such as ethylene (but could be lots of things, for example, alcohol vapor) is introduced. At these temperatures, the gas partially decomposes and/or rearranges into carbon-containing fragments and other molecules.
- These fragments and molecules then work their way onto the catalyst nanoparticles, where they stick and then break down into carbon. In this regard the nanoparticles act as mini catalytic converters, like the ones used in cars.
- At that point, the carbon dissolves (or something) into or on the nanoparticle (no one really knows for sure). This process is sort of like making rock candy, where the nanoparticle is a pot of hot water and the carbon is our sugar. In making rock candy, when a critical concentration of sugar is reached, addition of just a little bit more sugar causes the sugar dissolved in the hot water to precipitate out as a crystal. Same thing with nanotubes. Once a critical concentration of carbon is reached in the nanoparticle, addition of just a little bit more carbon from the vapor causes carbon to precipitate out in a crystal.
- When carbon crystallizes under atmospheric pressure (like in CVD), it crystallizes into flat sheets of hexagonally-patterned carbon. Stacks of these sheets are called graphite. But because the nanoparticle is so small and has such a high curvature, when the carbon crystallizes out it is constrained into a cylindrical shape. This is a carbon nanotube.
- As additional carbon gets fed into the nanoparticle, the nanotube continues to extrude out and grows longer.
- Nanotube growth stops when the carbon-containing gas is turned off, if the substrates cools down, or from a number of other things that can happen to the nanotube or catalyst nanoparticle.
Stuff You Can Grow Carbon Nanotubes On
You can grow nanotubes on really anything that can withstand the temperatures required for nanotube growth that doesn’t react with the catalyst nanoparticles in a detrimental way. Silicon wafers covered with catalyst nanoparticles are often used for nanotube growth, but ceramics, stainless steel, and aerogels make nice substrates too!
What Are Carbon Nanotubes Used For?
Carbon nanotubes have found use in a bunch of different applications:
- Strengthening composites
- Improving the electrical conductivity of plastics
- Large flat screen displays using field-emission
- Improving the product life of lithium ion batteries
- Nanoelectronics such as field-effect transistors
- Sensors for biomolecules
- MEMS devices
- Cancer therapies, where the nanotubes help deliver drugs to cancerous cells or are heated up by radio waves causing cells to explode
Aerogels and Nanotubes
This section describes two ways that aerogels and nanotubes intersect:
- Aerogels That Help Grow Carbon Nanotubes, and
- Aerogels Made Out of Carbon Nanotubes
Aerogels That Help Grow Carbon Nanotubes
Aerogels as Catalysts for Carbon Nanotubes
Aerogels are useful for carbon nanotube growth for two reasons.
Metal Oxide Aerogels as Nanotube Catalyst Supports
High-surface-area metal oxide aerogels of silica and alumina are ideal substrates for putting catalyst nanoparticles on. Defective, amorphous, high-surface-area, hard-to-reduce metal oxides seem to enhance the catalytic activity of catalyst nanoparticles used for growing carbon nanotubes. You see, catalyst nanoparticles are kind of like popcorn kernels-they don’t all “pop” at the same time (that is, they don’t all grow nanotubes at the same time), and some of them just never do. A metal oxide “support” like a silica aerogel or an alumina aerogel helps ensure a higher percentage of nanoparticles actually end up catalyzing nanotubes.
Metal Oxide Aerogels as Nanotube Catalysts Themselves
Since you can make aerogels out of iron oxide or with iron oxide mixed in, and aerogels are made out of a bunch of nanoparticles connected together, and nanoparticles of iron oxide can be used to make catalyst nanoparticles for growing carbon nanotubes, you can actually grow nanotubes directly on metal oxide aerogels, where the aerogel itself contains the nanoparticle seeds needed to catalyze carbon nanotube growth.
This has been done zillions of different ways. In general, a hybrid “mixed-matrix” gel of an inert metal oxide (like alumina) and iron oxide is made. The gel is supercritically dried into a mixed-matrix alumina-iron oxide aerogel. The aerogel is the used as the substrate for CVD. Localized pockets of iron oxide convert into iron nanoparticles that can then catalyze carbon nanotube growth! Prof. Jie Liu at Duke University has done a lot of work in this area.
Reference: B. Zheng, Y. Li, J. Liu, “CVD synthesis and purification of single-walled carbon nanotubes on aerogel-supported catalyst”, Applied Physics A, 74, 345-348 (2002).
Aerogels: Enhancing Carbon Nanotube Growth Since 1994
The first report describing a combination of aerogels and carbon nanotubes appeared in the Journal of Non-Crystalline Solids in 1994 by Aerogel.org contributors Dr. Arlon Hunt and Dr. Mike Ayers and their associate Dr. Wanqing Cao at Lawrence Berkeley National Laboratory.
In their work, CVD (they call it “chemical vapor infiltration”) of carbon into the pores of silica aerogels and silica aerogels doped with iron or nickel was explored. Although the paper focused on deposition of uniform layers of carbon into silica aerogels, they describe the appearance of “carbon rings, fibers, and tubes” as seen under transmission electron microscopy (TEM) in silica aerogels after CVD with a flow of 25% acetylene 75% argon at a rate of 100-300 sccm (standard cubic centimeters per minute) at temperatures of 550-580°C. They describe that the decomposition of the carbon-containing gases they used occurred at much lower temperatures in the presence of silica aerogels than without, indicating that the silica aerogel catalyzes decomposition of the gas. This paper is interesting because it’s not clear that if the nanotubes they observe grow from catalyst nanoparticles or if they just seem to self-assemble from confinement.
Reference: Arlon J. Hunt, Michael R. Ayers, Wanqing Cao, “Aerogel composites using chemical vapor infiltration”, Journal of Non-Crystalline Solids, 185, 227-232 (1995).
Growth of Nanotubes on Carbon Aerogels
In 2005, Stephen Steiner at MIT and Dr. Ted Baumann at Lawrence Livermore National Laboratory were researching Fe-doped carbon aerogels, essentially, carbon aerogels with iron nanoparticles dispersed throughout like blueberries in a blueberry muffin made of carbon. Steiner was growing bored of characterizing these aerogels and happened to work in a laboratory that also did CVD growth of carbon nanotubes. One day he wondered what would happen if he tried sticking an Fe-doped carbon aerogel into the CVD reactor, and surprisingly the aerogel came out coated with a grass-like layer of carbon nanotubes. It turned out that the Fe nanoparticles in the carbon aerogels acted as suitable catalysts for carbon nanotube growth. Nanotubes grown on Fe-doped carbon aerogels only penetrate about one micron into the porosity of a monolith due to limited diffusion through the tortuous mesopore network. Using a templating technique, however, he prepared macroporous monoliths of mesoporous Fe-doped carbon aerogels with plenty of room for diffusion and demonstrated that by opening up the pore network for gases to diffuse into, carbon nanotubes could be grown through the entire interior of a carbon aerogel monolith. Steiner later made carbon aerogels doped with nanoparticles of many other metals and metal oxides and discovered a number of never-before known nanotube catalysts.
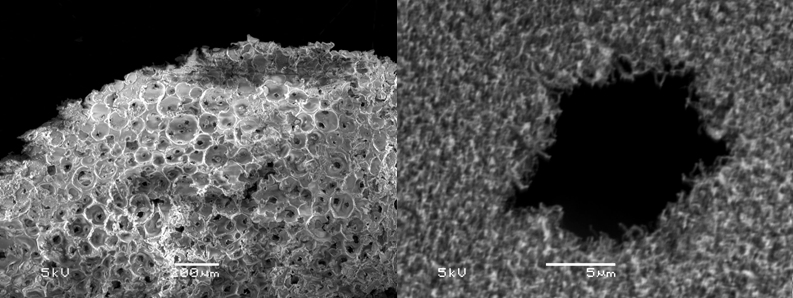
Templated porous Fe-doped carbon aerogel designed to enable growth of carbon nanotubes throughout its interior (left). Carbon nanotubes grown in a pocket of the aerogel (right).
References:
Stephen A. Steiner III, Theodore F. Baumann, Jing Kong, Joe H. Satcher, Jr., and Mildred S. Dresselhaus, “Iron-Doped Carbon Aerogels: Novel Porous Substrates for Direct Growth of Carbon Nanotubes”, Langmuir, 23, 9, 5161-5166 (2007).
Stephen A. Steiner III, “Engineering Carbon Nanostructures: Development of Novel Aerogel-Nanotube Composites and Optimization Techniques for Nanotube Growth”, S.M. Thesis, Department of Materials Science and Engineering, Massachusetts Institute of Technology, May 31, 2006.
Aerogels Made from Carbon Nanotubes
Carbon Nanotube Aerogels
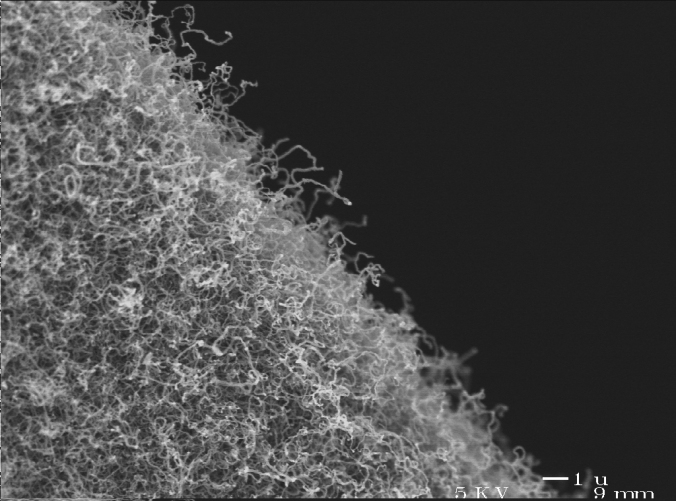
In 2007, Mateusz Brying et al. working with Prof. Arjun Yodh at the University of Pennsylvania produced the first aerogels made entirely of carbon nanotubes. In their work, powdered carbon nanotubes were dissolved in water using the surfactant sodium dodecylbenzenesulfonate (that is, they basically dissolved the carbon nanotubes with soap and water) producing a solution with a concentration of carbon nanotubes of 5-13 mg/mL. The solutions are poured into a mold and gel overnight into an elastic gel. The gels were then solvent exchanged into either water or solutions of 0.25-1 wt % polyvinyl alcohol, then into ethanol, and finally into liquid CO2 and supercritically dried. The result was carbon nanotube aerogels, with less than 5% shrinkage.
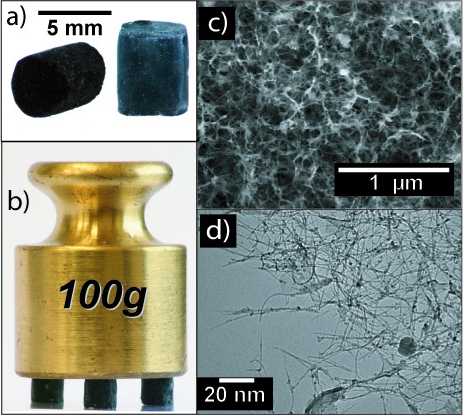
Carbon nanotube aerogels (a) as produced, (b) holding 8000 times their weight, (c) under scanning electron microscopy, and (d) under transmission electron microscopy. (Image from Mateusz B. Bryning, Daniel E. Milkie, Mohammad F. Islam, Lawrence A. Hough, James M. Kikkawa, Arjun G. Yodh, "Carbon Nanotube Aerogels", Advanced Materials, 19, 5, 662 (2007). Copyright Wiley-VCH Verlag GmbH & Co. KGaA. Reproduced with permission. Not open source.)
These aerogels have densities of about 0.01-0.06 g cm-3, but as low as 0.0075 g cm-3 (!) and can hold 8000 times their weight in applied force. They contain pores ranging from tens of nm to microns, with a unique filamentous structure (as opposed to the string of pearls morphology). Polyvinyl alcohol treatment before supercritical drying creates a conformal coating of “glue” that covers the surfaces of the nanotubes and holds the aerogel together better.
One of the most promising applications of these materials is for electrical applications. They have remarkably high electrical conductivities for their low densities compared to similar density carbon aerogels-as high as 0.7 Ω-1 cm-1 for a 0.0075 g cm-3 nanotube aerogel, equivalent to a 0.06 g cm-3 carbon aerogel (eight times heavier)! This is important since there are many applications where you want to have a high surface area electrical conductor, but to get higher surface areas you usually have to decrease the density of the aerogel, which means decreasing electrical conductivity.
References:
Mateusz B. Bryning, Daniel E. Milkie, Mohammad F. Islam, Lawrence A. Hough, James M. Kikkawa, Arjun G. Yodh, “Carbon Nanotube Aerogels”, Advanced Materials, 19, 5, 662 (2007).
Xianping Lu, Ove Nilsson, Jochen Fricke, Richard W. Pekala ,”Thermal and electrical conductivity of monolithic carbon aerogels”, Journal of Applied Physics, 73, 2, 581-584 (Jan 1993).
Carbon-Carbon Nanotube Aerogels
In 2008, Dr. Ted Baumann and Dr. Marcus Worsley at Lawrence Livermore National Laboratory developed a composite material made of double-wall carbon nanotubes dispersed uniformly throughout a carbon aerogel. In their process, double-wall carbon nanotubes are dissolved in water again with the surfactant sodium dodecylbenzenesulfonate. Resorcinol and formaldehyde are then added to this solution along with sodium carbonate catalyst to form a resorcinol-formaldehyde polymer. The gel is cured at 85°C, exchanged into acetone and then liquid CO2 and finally supercritically dried. The RF-nanotube hybrid aerogels are then pyrolyzed under nitrogen at 1050°C. The resulting carbon-carbon nanotube aerogels have improved electrical conductivities over regular carbon aerogels. For 8 wt % nanotube loading and bulk density of 0.139 g cm-3, these aerogels have about twice the conductivity of regular carbon aerogels (5 Ω-1 cm-1 vs. 2 Ω-1 cm-1).
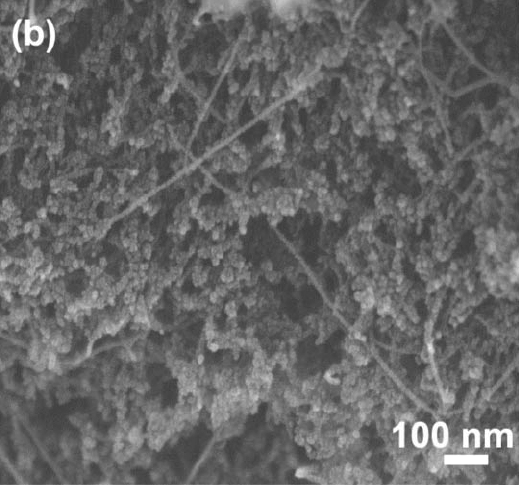
Carbon aerogels with double-wall carbon nanotubes dispersed inside. (Reproduced from Marcus A. Worsley, Joe H. Satcher Jr., and Theodore F. Baumann, "Synthesis and Characterization of Monolithic Carbon Aerogel Nanocomposites Containing Double-Walled Carbon Nanotubes", Langmuir, 24, 17, 9763-9766 (2008). Copyright 2008 American Chemical Society. Not open source.)
In an exciting variation on this, carbon-carbon aerogels with higher nanotube loadings (~90% carbon nanotubes) are remarkably elastic, and can be compressed up to 80% and fully recover.
Reference:
Marcus A. Worsley, Joe H. Satcher Jr., and Theodore F. Baumann, “Synthesis and Characterization of Monolithic Carbon Aerogel Nanocomposites Containing Double-Walled Carbon Nanotubes”, Langmuir, 24, 17, 9763-9766 (2008).
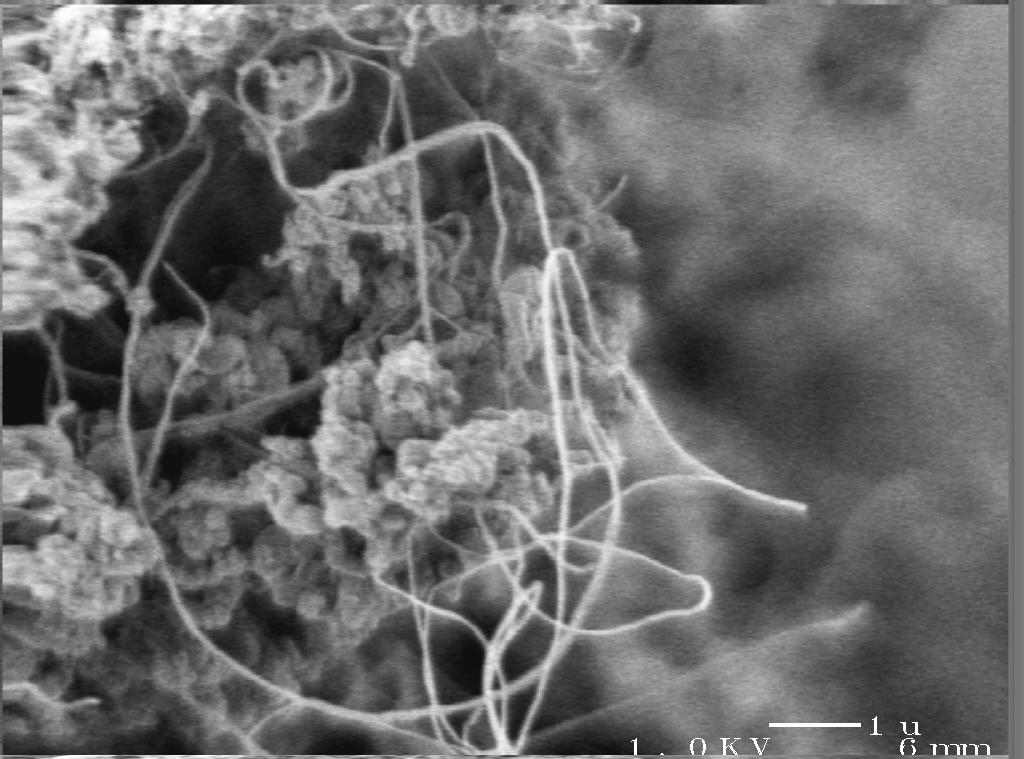
Nanotube “Aerogels” in Nanotube Fiber and Sheet Production
Carbon nanotube yarns and sheets are an exciting area of research. These are materials made completely of entangle carbon nanotubes.
Unfortunately, the term “aerogel” has been used to describe smokes of carbon nanotubes which form during production of nanotube yarns and even thin sheets of carbon nanotubes drawn from carbon nanotube forests. This is not an appropriate use of the word aerogel, since these materials do not have defined mesoporosity and especially in the case of nanotube smokes, are generally not monolithic materials. The terms “elastic smoke” or “ethereal film” is more appropriate.
Nonetheless, these are interesting materials and are worth reading more about themselves. Read more at: