Pyrolysis
Materials
- An organic aerogel, such as a resorcinol-formaldehyde aerogel (see the recipe called Organic Aerogels under the Make section) or other suitable precursor aerogel
- Nitrogen or argon gas tank equipped with a regulator with a 30 psi output max
and
- High-temperature (1100°C max) tube furnace such as a Lindberg Blue/M Mini-Mite
- Fused quartz process tube
- End caps to connect gas tank to quartz tube and to connect other end of quartz tube to exhaust line
or alternatively,
- A high-temperature box furnace or oven equipped with a thermometer
- Ceramic crucible
- Pipe fitting to adapt gas tank output to furnace input
- Exhaust line out of furnace
Optional
- Mass flow meter or controller
Overview
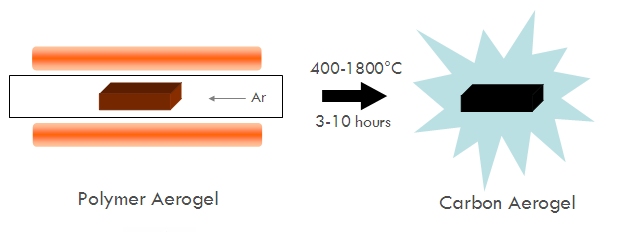
Some aerogels, like carbon aerogels, are derivative or secondary aerogels, that is, they are derived by processing already existing aerogels. For example, carbon aerogels are typically prepared by pyrolyzing organic aerogels such as resorcinol-formaldehyde aerogels in an inert atmosphere.
Aerogel Preparation
- Prepare a suitable precursor aerogel (to make carbon aerogels see the recipe for making Organic Aerogels for a suitable resorcinol-formaldehyde precursor aerogel).
- Place the aerogel into the center of the quartz tube or crucible.
- If using a tube furnace, place the quartz tube in the center of the tube furnace such that the aerogel is halfway along the heated length. If using a box furnace, place the crucible in the center of the furnace where possible.
- Connect the nitrogen or argon line. If using a quartz tube, slide and secure the end cap connected the gas tank onto one end of the tube. If using a box furnace, attach the gas supply line to a suitable, sealed fitting (such as a barb-to-NPT adapter) attached to the furnace.
- Connect an exhaust line to your system and route the exhaust into a suitable vent such as an exhaust duct or a chemical fume hood.
- Open the gas tank. Open the regulator to allow gas to flow. A suitable flow rate for a 1″ quartz tube is 200 sccm (standard cubic centimeters per minute) of gas. If not using a mass flow meter, this should be a few psi and a relatively gentle flow (about what you would use to blow bubbles in a glass of milk).
- Set the furnace temperature between 600 and 1050ºC (although temperatures of 400-1800ºC will work, this is the common range). The temperature you set will determine the degree of pyrolyzation (for organic aerogels, the degree of carbonization and/or graphitization).
- Once at temperature, allow the aerogel to pyrolyze for 3-10 h. Important: If your system takes a long time to ramp to its temperature set point, you will need to factor this into the total pyrolysis time to get desired results. A fast-heating furnace like a Mini-Mite will take minutes to reach temperature and require longer soak times at the set point than a large box furnace that takes 1-3 hours to ramp (since the aerogel will be above pyrolyzable temperatures during much of the ramp phase).
- Turn the furnace off. Wait until the furnace has cooled to ~200ºC or less before opening.
- Turn gas tank off.
What’s Happening
In the case of carbon aerogels, the high temperature causes the organic polymer to dehydrate, releasing water vapor, carbon monoxide, and carbon dioxide leaving behind nanocrystalline (effectively amorphous) carbon. If this is done in air the polymer will simply burn. To guarantee pyrolysis occurs, an atmosphere of nitrogen or argon or other inert gas is needed.
What Doesn’t Work
- Using any old polymer aerogel. Most organic aerogels are resorcinol-formaldehyde or melamine-formaldehyde based and these all work. Other polymer aerogels aren’t guaranteed to.
- Not using an inert atmosphere. Your aerogel will burn away. It is sometimes necessary, however, to oxidize some polymers such as poly(acrylonitrile) at a low temperature (300-400ºC) prior to pyrolyzing in an inert atmosphere.
Variables You Can Play With
- Adjusting the flow rate will change the degree of pyrolyzation. Higher flow rate will result in a cooling effect and may require longer pyrolysis times to get the same results as using a low flow rate.
- Changing the time and temperature of the process will change the degree of pyrolysis. For some aerogels, this may affect morphology, surface area, and density of the resulting derivative aerogel.
What You Should Get
A pyrolyzed, derivative aerogel such as a carbon aerogel. In the case of carbon aerogels, you will find that the aerogel will have shrunk significantly in comparision with the precursor aerogel; however, the aerogel will also have lost proportionately about the same amount of mass from dehydrating. Thus, the resulting density will be about the same as the original precursor aerogel.

Sir, i need few papers on use of carbon aerogels in water treatment and wastewater treatment. I do not have access to journals since this facility is not available in our college. Kindly help me…
Dear Sir,
I want the same carbon aerogel with that thickness which you have shown in your picture for our waste water treatment. If you sell the carbon aerogel, it’s fine, I will get it from you. Otherwise, please let me know the supplier of carbon aerogel, so that I can purchase from them.
Looking forward to hear from you.
Please reply me by email.
Thanks and Regards,
P.Gayathri,
Project Engineer.
Flagship Eco Systems Pte Ltd,
4 Shenton way, #06-01, SGX Center 2,
Singapore – 068807.
PH :+65-65361110
Fax : +65-65365550
Hi Gayathri,
Good question–you can try Aerogel Technologies who makes a wide range of aerogel materials or alternatively Marketech International who sells some carbon aerogel materials.
hello stephen. i’ve try to make carbon aerogel with your recipe but i got one trouble. when i pyrolisis my organic aerogel, my organic aerogel burn on 300ºC. would you help, how can be like that. thank’s for your kindness. i’ll wait your answer
Hi Fajran,
Sorry to hear that! The most common reason an organic aerogel would burn away at 300 deg C is because of an oxygen leak, or possibly because you’re not using an inert gas such as nitrogen or argon to do the pyrolysis. Make sure you are not doing the pyrolysis in air and, if you are using nitrogen or argon, make sure there is no leak in your system that is allowing oxygen or water vapor in.
respected Sir,
Can you explain me that whether the Resorcino- Formaldehyde aerogels could be used for hydrogen storage experiments and what if carbon aerogels could be used.
after pyrolyzind RF aerogel whether teh porosityand surface area of the carbon aerogel will remain with slight effect.
please let me know.
Thanking you.
Hi Pradeep,
Usually for hydrogen storage experiments people look at carbon aerogels (the pyrolyzed derivatives of resorcinol-formaldehyde aerogels), or sometimes carbon or silica aerogels doped with metal nanoparticles. Carbon aerogels do retain most of the surface area of resorcinol-formaldehyde aerogels and, in fact, can be further processed to have much higher surface area than the original organic aerogels.
Hi Szymon,
The only difference between calcination and pyrolysis is what’s happening to the material. Calcination is also usually done in air. But from a process standpoint they are very similar.
Tube furnaces are pretty common where they do materials science with metals or CVD. You can use a box furnace (check eBay) or even take a quartz tube, wrap it with Ni-Cr wire or equivalent, hook it up to a power supply, and make your own mini tube furnace. Probably a DC power supply putting out 20 A or so would be enough–the voltage will depend on the wire you use. You can buy something like that for about $200-$300 on Digikey.
If you build your own I would make sure to have a good stand and some high-temperature insulation around the heating wire (maybe Pyrogel from BuyAerogel.com?) to make sure it’s safe.
Sounds like a good do-it-yourself project to contribute to Aerogel.org!
1. What is the difference between this process and calcination, which is used, as far as I know, to remove water from metal oxide aerogels that are hydrated to some extent?
2. Can I use something else than tube furnace? I don’t think this is an easy to find equipment.
Regards,
Szymon